
Zinc phosphate is a versatile compound with multiple variations, each characterized by a distinct chemical formula. Let's explore these different formulas and understand their significance in various chemical contexts.
This article explains Zinc Phosphate formula, also known as Trizinc Phosphate formula and Trizinc diphosphate formula. Zinc Phosphate is an inorganic compound that is used as a corrosion-resistant coating for metal surfaces. It is used either as a primer pigment or in electroplating. Zinc Phosphate has the molecular or chemical formula Zn3(PO4)2.
Trizinc phosphate is obtained from zinc phosphate cement, which is widely used in dentistry as a base for dental restorations.
What is Zinc Phosphate?
The inorganic compound Zn3(PO4)2, also known as Zinc Phosphate, is a versatile white powder often used as a corrosion-resistant metal surface coating. Its other names include Zinc orthophosphate, Trizinc phosphate, and Trizinc diphosphate. This coating is commonly applied through electroplating or as a primer pigment, providing superior protection compared to untreated metal due to its crystalline structure. A seeding agent such as sodium pyrophosphate is frequently used as a pre-treatment to enhance its effectiveness. In fact, the use of toxic materials like chromium or lead has largely been replaced by this safer alternative. As of 2006, Zn3(PO4)2 has become one of the most widely employed corrosion inhibitors. The main ingredient in this compound is sodium pyrophosphate.
Zinc Phosphate Formula
Zinc phosphate, in a more general sense, is often represented by the chemical formula Zn3(PO4)2. This formula reflects the combination of zinc ions (Zn^2+) and phosphate ions (PO4^3-). The chemical formula highlights the ionic nature of the compound, where positively charged zinc ions and negatively charged phosphate ions come together through electrostatic attraction.
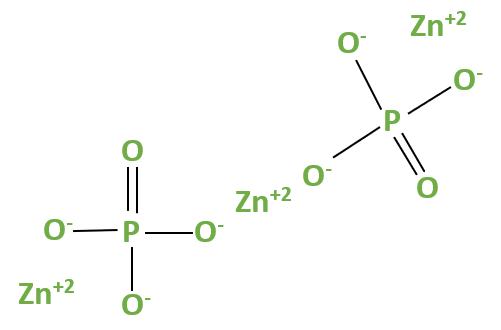
Zinc Phosphate Formula by Criss Cross Method
The criss-cross method is a simple way to determine the chemical formula of an ionic compound like zinc phosphate. It involves swapping the valencies of the cation (in this case, zinc) and the anion (phosphate). In zinc phosphate, the formula is Zn3(PO4)2. This formula signifies that three zinc ions (Zn^2+) combine with two phosphate ions (PO4^3-) to form the compound.
Zinc Phosphide Formula
Zinc phosphide is a different compound with the chemical formula Zn3P2. Unlike zinc phosphate, which contains phosphate ions, zinc phosphide consists of zinc cations (Zn^2+) and phosphide anions (P3-).
Occurrence of Zinc phosphate
Zinc orthophosphate exists in several forms, such as minerals like parahopeite and hopeite, as well as a mineral called tarbuttite (Zn2(PO4)(OH)), which is derived from zinc phosphate cement. This type of cement is widely used in dentistry for various purposes including as a base for dental restorations, temporary restorations, and for orthodontic appliances. It is composed of a mixture of zinc oxide and magnesium oxide powders combined with a liquid made of phosphoric acid, water, and buffers.
Zinc Hydrogen Phosphate Formula
Zinc hydrogen phosphate, also known as zinc dihydrogen phosphate, is represented by the chemical formula Zn(H2PO4)2. This compound consists of zinc cations (Zn^2+) and dihydrogen phosphate ions (H2PO4^-).
Zinc Dihydrogen Phosphate Formula
Zinc dihydrogen phosphate has the same formula as zinc hydrogen phosphate: Zn(H2PO4)2. This compound contains zinc ions and dihydrogen phosphate ions.
Zinc II Phosphate Formula
The Roman numeral "II" in "Zinc II Phosphate" indicates that zinc is in its +2 oxidation state. The formula for zinc II phosphate is Zn3(PO4)2. This compound, like zinc phosphate, consists of zinc ions (Zn^2+) and phosphate ions (PO4^3-).
Zinc Ammonium Phosphate Formula
The formula for zinc ammonium phosphate, which is also known as ammonium zinc phosphate, is (NH4)3Zn4(PO4)3. This compound contains ammonium ions (NH4+), zinc ions (Zn^2+), and phosphate ions (PO4^3-).
Zinc Phosphate Tetrahydrate Formula
The formula for zinc phosphate tetrahydrate is Zn3(PO4)2·4H2O. This compound is a hydrated form of zinc phosphate, indicating the presence of four water (H2O) molecules for every unit of zinc phosphate.
These different zinc phosphate formulas and related compounds reflect the versatile nature of zinc chemistry and its ability to form a wide range of compounds with distinct properties and applications.
Zinc Phosphate properties
A solution of magnesium oxide and zinc oxide powders is mixed with water, phosphoric acid, and buffers to create Zinc Phosphate Formula. It is one of the most established cement types to compare against in terms of dental history. However, resin-modified glass ionomer cements are more robust and practical when used in dental settings, even though they are still widely used.Physical Properties of Zinc Phosphate
- A white crystal of Zinc Phosphate Formula is how it is found in nature.
- The zinc Phosphate Formula has a molecular weight of 386.11 g/mol, a density of 3.998 g/cm3,
- Zinc Phosphate melting temperature of 900°C.
- This substance is inflammable.
Chemical Properties of Zinc Phosphate
- Water does not dissolve the Zinc Phosphate Formula.
- Zinc Phosphate Formula is applied to metal surfaces because of its corrosion resistance.
Uses of Zinc Phosphate
- The zinc Phosphate Formula is used to produce colours.
- In addition to serving as a surface treatment agent, Zinc Phosphate Formula is also used as a plating agent.
- The zinc Phosphate Formula is commonly used to prevent corrosion.
- Paints contain it as a component.
- The Zinc Phosphate Formula is used to treat water.
- Scaling is inhibited by it.
- Rubber goods are made from it.
| Related Links | |
| Pentane formula | Octane formula |
| Oxalic Acid formula | Nickel Chloride formula |
Zinc Phosphate Formula FAQs
What is a Zinc Phosphate called?
What is the formula for zinc permanganate?
How do you name zinc phosphide?
What is the chemical formula of Zinc Phosphate Zn2+ and PO4^3−?










