
The Pentane Formula is C5H12. It comprises five carbon atoms (C) and twelve hydrogen atoms (H). Pentane Formula is an alkane hydrocarbon and is one of the most straightforward hydrocarbons in its molecular composition.
Pentane is a hydrocarbon compound widely used as a reference in the field of organic chemistry. Its molecular formula, C5H12, represents a straightforward structure composed of carbon (C) and hydrogen (H) atoms.Pentane Formula
The pentane formula is an aliphatic hydrocarbon belonging to the alkane family. Its molecular formula, C5H12, signifies that it comprises five carbon atoms and twelve hydrogen atoms. Pentane is one of the simplest and most fundamental hydrocarbons, featuring a straight-chain structure with five carbon atoms bonded together in a linear arrangement, each bonded to two or three hydrogen atoms.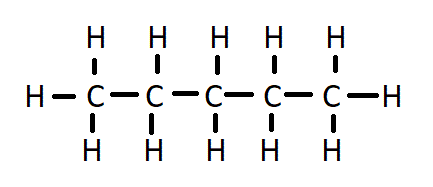
Pentane Formula Structure
The structural formula of pentane can be depicted as follows: In this representation, each "C" represents a carbon atom, and each "H" represents a hydrogen atom. Pentane's linear, unbranched structure is a hallmark of the aliphatic hydrocarbons.
In this representation, each "C" represents a carbon atom, and each "H" represents a hydrogen atom. Pentane's linear, unbranched structure is a hallmark of the aliphatic hydrocarbons.
Also Check - Aspirin Formula
Pentane Formula Chemical
Pentane, with its molecular formula C5H12, belongs to the alkane family, which consists of saturated hydrocarbons. As an alkane, it undergoes combustion reactions, producing carbon dioxide (CO2) and water (H2O) as products. Its chemical properties make it useful as a fuel, particularly in laboratories where it is a clean-burning, readily available heat source.Pentane Empirical Formula
A compound's empirical formula expresses its elements' simplest whole-number ratio. For pentane (C5H12), the empirical formula is CH2. This ratio indicates that for every five carbon atoms, there are twelve hydrogen atoms in the compound.Pentane Condensed Formula
In a condensed structural formula, pentane is represented as C5H12 without explicitly showing all the bonds between atoms. It provides a more compact way to represent the molecule, especially for larger or more complex compounds.Pentane Condensed Formula: CH3(CH2)3CH3
Here, the "CH3" group represents a methyl group (CH3) attached to the central carbon atom of the pentane molecule. The "CH2" units represent methylene (-CH2-) groups, and the "CH3" at the end indicates another methyl group. Pentane, with its molecular formula C5H12, is a simple yet essential hydrocarbon in organic chemistry. Its linear structure and straightforward chemical properties make it a valuable reference compound and a key player in various chemical processes, including combustion reactions and as a laboratory heat source.Isomers of Pentane Formula
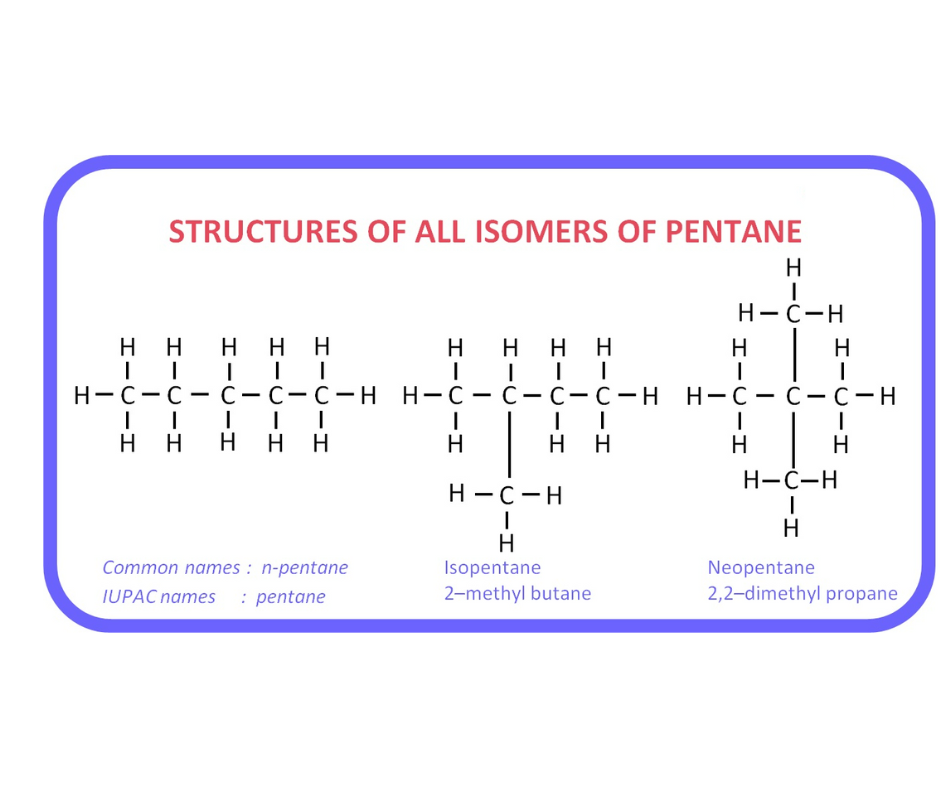 Pentane, with the chemical formula C5H12, can exist in three distinct structural isomers. These isomers have different arrangements of carbon atoms in their molecular structures, resulting in unique physical and chemical properties. The three isomers of pentane are:
Pentane, with the chemical formula C5H12, can exist in three distinct structural isomers. These isomers have different arrangements of carbon atoms in their molecular structures, resulting in unique physical and chemical properties. The three isomers of pentane are:
n-Pentane (Normal Pentane)
- Molecular Structure: CH3CH2CH2CH2CH3 - Description: This is the straight-chain isomer of pentane, where the carbon atoms form a continuous chain with no branching. It is often referred to as "normal pentane."Isopentane (2-Methylbutane)
- Molecular Structure: (CH3)2CHCH2CH3 - Description: Isopentane has a branched structure with a methyl (CH3) group attached to one of the carbon atoms in the chain. This branching gives it its common name, "isopentane."Neopentane (2,2-Dimethylpropane)
- Molecular Structure: (CH3)3CCH3 - Description: Neopentane is the most highly branched isomer of pentane, featuring three methyl (CH3) groups attached to a central carbon atom. It is also known as "2,2-dimethylpropane." These three isomers of pentane have different physical properties such as boiling points, which vary due to their structural differences. Each isomer plays a distinct role in various chemical and industrial applications, making them important compounds in the field of organic chemistry.| Related Links | |
| Cyanide Formula | Sulfurous Acid formula |
| Resorcinol Acid formula | Dinitrogen Trioxide Formula |
Pentane Formula FAQs
Is pentane 5 or 6?
Pentane is a hydrocarbon compound with five carbon atoms (C5) in its molecular structure. The prefix "pent-" in its name signifies the presence of five carbon atoms.
What is pentane used for?
Pentane has several applications, primarily as a laboratory solvent and a clean-burning fuel. It is used as a heat source in laboratories, especially for gas chromatography and as a fuel for camping stoves. Additionally, it is employed in the production of certain chemical compounds and as a blowing agent in the manufacture of foam plastics.
Is pentane 5 carbons?
Yes, pentane consists of five carbon atoms (C5) in its molecular structure. The prefix "pent-" denotes the number five.
What type is pentane?
Pentane is an aliphatic hydrocarbon, specifically an alkane. It is a saturated hydrocarbon characterized by single bonds between carbon atoms, forming a straight-chain structure. This straight-chain arrangement classifies it as a normal or n-pentane.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









