
Octane Formula is C8H18. Octane, a hydrocarbon known for its significance in the world of fuel, has a unique chemical formula. The chemical formula for octane is C8H18. Octane formula represents its molecular composition: eight carbon atoms (C) and eighteen hydrogen atoms (H). Octane is a saturated hydrocarbon, specifically an alkane, and is known for its use as a fuel additive in gasoline to improve engine performance and reduce engine knock in internal combustion engines.
Octane Formula
The chemical formula for octane is C8H18. This formula encapsulates its molecular composition, which consists of eight carbon atoms (C) and eighteen hydrogen atoms (H). Octane is a saturated hydrocarbon, belonging to the alkane family, and is well-known for its combustible properties.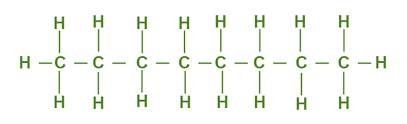
Octane Formula Structure
The structure of octane is relatively straightforward, reflecting its saturated hydrocarbon nature. It is an elongated carbon chain with eight carbon atoms, each bonded to two hydrogen atoms, and linked by single covalent bonds. The structure can be depicted as follows: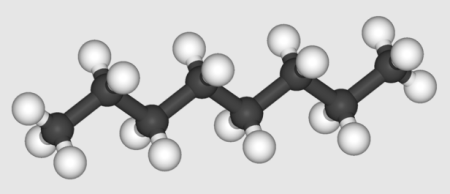
Also Read: Hydrocarbon Formula
Octane Formula Molar Mass
The molar mass of octane is the sum of the atomic masses of the constituent carbon and hydrogen atoms in the molecular formula. To calculate the molar mass of octane: - 8 carbon atoms (C), each with an atomic mass of approximately 12.01 g/mol. - 18 hydrogen atoms (H), each with an atomic mass of approximately 1.01 g/mol. Molar Mass of Octane = (8 * 12.01 g/mol) + (18 * 1.01 g/mol) ≈ 114.18 g/mol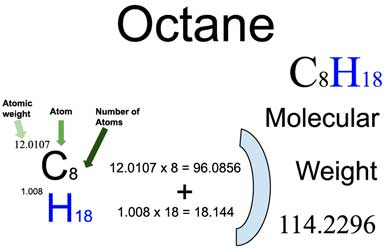
Also Read: Amines Formula
Octane Formula Combustion
Octane is renowned for its use as a standard reference in the octane rating, which measures the performance of gasoline in internal combustion engines. The combustion of octane is a fundamental process in engine operation. Octane's high energy content and clean combustion make it a preferred choice for gasoline, powering the world's vehicles.Octane Formula in Gasoline
Octane is a vital component in gasoline, often referred to as "octane fuel." Gasoline blends containing higher percentages of octane exhibit better anti-knock properties, allowing for smoother and more efficient engine operation. It plays a pivotal role in enhancing engine performance and reducing engine knock, a phenomenon where uncontrolled combustion occurs.Octane Formula Empirical
The empirical formula of octane, which represents the simplest whole-number ratio of the elements in the compound, is CH2. This ratio is derived from the molecular formula (C8H18) by dividing both carbon and hydrogen by their greatest common factor, which is 2.Octane Formula and Charge
Octane is a neutral molecule, and as such, it carries no overall electric charge. The equal number of positively charged protons in the nucleus and negatively charged electrons in the electron cloud results in a net charge of zero for the entire octane molecule.Octane Formula Compound
Octane is classified as an alkane hydrocarbon, a type of organic compound that consists of carbon-carbon single bonds and carbon-hydrogen bonds. It's one of the many hydrocarbons used as fuel sources due to its stable and combustible properties. The octane formula, C8H18, exemplifies its chemical structure and role as a critical component of gasoline, powering engines worldwide.Also Read : D Block
Properties of Octane
Octane, a hydrocarbon belonging to the alkane family, exhibits various physical and chemical properties that make it an essential component of gasoline and a reference standard for assessing fuel performance. Below are some of its key properties:| Properties of Octane | |
| Chemical formula of Octane | C 8 H 18 |
| Density | 114.232 g/mol |
| Chemical names | n-octane, 111-65-9 Oktan, Oktanen |
| density | 0.703 g cm−3 |
| Boiling point of Octane | 125.1 to 126.1 °C |
| Melting point of Octane | −57.1 to −56.6 °C |
Application of Octane
Reducing octane prevents the air-fuel mixture from igniting before the spark plug ignites it. An engine's maximum power is provided by sparking or firing the air-fuel mixture at the right time. Using gasoline with a higher octane number is only a waste of money than it is designed for.| Related Links | |
| Polymers | Avogadro Law |
| Solid state | Aluminium Nitrate Formula |
Octane Formula FAQs
What is octane formula in Class 10 chemistry?
Octane is typically represented by its molecular formula, which is C8H18. This formula signifies its chemical composition and structure as a hydrocarbon.
What is octane in chemistry?
In chemistry, octane is a hydrocarbon, specifically an alkane. It's a saturated hydrocarbon consisting of eight carbon atoms and eighteen hydrogen atoms, with the molecular formula C8H18. Octane is commonly found in gasoline and is known for its role in controlling engine knock in internal combustion engines.
What is octane used for?
Octane is primarily used as a fuel additive and a reference standard in the automotive and energy industries. It's a crucial component of gasoline, where it helps control engine knock and improve engine performance. Octane is also used in laboratory settings to assess the octane rating of fuels.
What is octane in fuel?
Octane in fuel refers to the octane rating, which is a measure of a fuel's resistance to engine knock in internal combustion engines. Fuels with higher octane ratings, like octane itself, are less prone to knocking. Octane is used in gasoline to enhance engine performance and reduce engine knock, ensuring smoother and more efficient engine operation.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









