
To understand the Freezing Point Depression Formula , lets first learn about freezing. The phase transition of a material from a liquid state to a solid state is referred to as freezing. The phenomenon is known as a phase transition, which occurs when a material changes from one state of matter to another. The solid and liquid states coexist at the freezing point because these two phases, liquid and solid, are in balance.
The atmospheric pressure influences the freezing point. Another primary factor that influences the freezing point is the presence of solutes. Adding salt or sugar in water and other solvents causes the freezing point to lower. This is called depression in the freezing point. We will discuss this phenomenon and its derivation and formulas in detail.Colligative Properties
The physical modifications brought about by mixing a solute with a solvent are referred to as colligative properties. Colligative properties are influenced by the amount of solvent and the number of solute particles present, but not by the kind of solute particles present, even if the kind of solvent influences them.Also Check - Bond Order Formula
Elevation in Boiling Point
There is a difference between the boiling points of pure solvents and those of solutions. According to the solute's molality, the effect is inversely proportionate. This implies that the addition of solute to the solvent causes the boiling point to be higher.Also Check - Charles Law Formula
Depression in the Freezing Point
All solutions have lower freezing points than the pure solvent. The molality of the solute has a direct correlation with the freezing point depression.Osmotic Pressure
The pressure difference required to stop solvent from flowing through a semipermeable barrier is known as the osmotic pressure of a solution. The molar concentration of the solute particles in a solution directly relates to the osmotic pressure of a solution.Reduced Vapour Pressure
A solvent's vapour pressure in a solution is always lower than the solvent's vapour pressure in pure form. The mole fraction of the solute has a direct relationship with the reduction in vapour pressure.Also Check - Chromium III Sulfate Formula
Chemistry Formula For Depression In Freezing Point
We are aware of the fact that water freezes into ice at temperatures below zero. However, when solutes or impurities like salt and sugar are present in water, its freezing point becomes lower than zero. This implies that the solution of salt and water remains in the liquid state even as the temperature approaches zero. This phenomenon is termed a lowering of the freezing point or depression in the freezing point. [caption id="attachment_17361" align="alignnone" width="694"]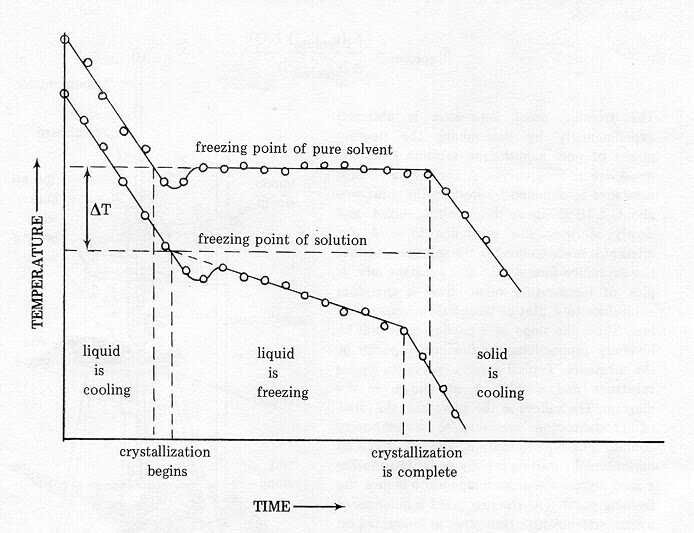 Chemistry formula for depression in freezing point[/caption]
Chemistry formula for depression in freezing point[/caption]
Causes of Depression in Freezing Point
Following is an explanation of why adding a solute causes a solvent's freezing point to decrease.- There is an equilibrium between a solvent's liquid and solid states at its freezing point.
- This suggests that the liquid and solid phases have the same vapour pressures.
- The vapour pressure of the solution is discovered to be lower than the vapour pressure of the pure solvent upon the addition of a non-volatile solute.
- As a result, the solid and the solution come into equilibrium at lower temperatures.
Also Check - Tungstic Acid formula
The Chemistry Formula for Depression in Freezing Point
The depression in the freezing point is expressed by the symbol Δ T f . This depression can be calculated with the formula Δ T f =i × K f × m . Here, Δ T f = Depression in the freezing point. i = Van’t Hoff factor K f = Cryoscopic constant and m= Molality of the substance As a consequence of the solute dissolving in the solution, the melting point is lowered in accordance with Raoult's law since the vapour pressure of a pure solvent lowers. A nonvolatile solvent, as far as we are aware, has no vapour pressure. The overall vapour pressure of the mixture is therefore lower than the vapour pressure of the pure solvent. Water, for instance, melts at zero degrees Celsius. With a drop in vapour pressure, a solvent's freezing point lowers and its vapour pressure is lower in a solution containing a non-volatile solute. When the vapour pressures of the two phases are equal, a dynamic equilibrium can exist between the solid and liquid phases.Applications of Chemistry Formula For Depression in Freezing Point
Colligative characteristics have real-world uses, such as when salting roads in cold climates. The melting point of the ice is lowered and will melt more quickly if salt is applied to an icy road, improving driving safety. The most often utilised salts are sodium chloride (NaCl) and either calcium chloride (CaC l 2 ) or magnesium chloride (MnC l 2 ) , either separately or in combination. The least costly alternative, sodium chloride, only dissociates into two ions as opposed to three, which makes it less effective. Ethylene glycol and water are usually used to make radiator fluids for different types of cars. In the winter, this helps prevent the radiator from freezing. The freezing point depression formula can be used to determine the molar mass of a certain solute. The same freezing point depression formula may be used to determine how much a solute can dissociate in a solvent. The word for this kind of measurement is cryoscopy, which literally translates to "observing the cold," and it depends on having a precise grasp of the freezing point. In the culinary industry, this phenomenon is employed to create sweets like ice cream by freezing a combination of salt and sugar.Factors Affecting the Freezing Point
The sort of molecules that make up the liquid are the key determining factors for the freezing point of a solution.- A material's high freezing point indicates strong intermolecular forces between its molecules.
- The freezing point is quite low if the forces are weak.
- When the solid and liquid phases are in balance, a liquid freezes or a solid melts.
Examples of Chemistry Formula For Depression in Freezing Point
- Seawater has a lower freezing point than 0 degrees Celsius. At temperatures below the freezing point of pure water, seawater is still a liquid. This is a result of the dissolved salts in the saltwater.
- A solution of ethanol in water is another typical example of this phenomenon. The mixture has a freezing point that is greater than pure ethanol but lower than pure water.
Freezing Point Depression Formula FAQs
What is the definition of the freezing point of a substance?
The freezing point of a liquid is the temperature, at which the liquid particles start to transform into a solid. The freezing point for water is 100 degree C.
Define the term depression in freezing point.
The term depression in freezing point refers to the lowering of the temperature at which a solvent usually freezes due to the addition of a solvent.
What is the freezing point depression constant for water?
Kf is equal to -1.86°C/m for water. So any nonvolatile molecular solute in a 1-molal aqueous solution will freeze at -1.86 °C. The molal freezing-point depression constant for each solvent is different.
What is the basis behind the freezing point depression?
Colligative properties also include the freezing point depression brought on by a solute. In other words, the degree of change in the freezing point depends on how many solute particles are present in a solution rather than how the solute is chemically composed.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









