
Typically, the term chromium III sulfate formula refers to inorganic substances having the formula C r 2 (S O 4 ) 3 .x( H 2 O) , where x can be any value between 0 and 18. Undefined but commercially significant "basic chromium sulfates" are another thing that is well recognised. These salts are often soluble in water solids that are either violet or green in colour. It is frequently employed while tanning leather.
There are various compounds of chromium that exist naturally and chromium III sulfate is one of them.Introduction to Chromium
The chemical element chromium has the atomic number 24 and the symbol Cr. It is group 6's initial element. It is a transition metal that is steely grey, glossy, tough, and brittle. The great corrosion resistance and hardness of chromium metal make it valuable. The discovery that steel could be rendered very resistant to corrosion and discolouration by adding metallic chromium to make stainless steel was a significant advancement in the manufacture of steel. 85% of commercial use is made up of stainless steel with chrome plating (electroplating with chromium). Chromium is highly prized for its ability to be finely polished while remaining tarnish-resistant. Almost 70% of the visible spectrum and almost 90% of infrared light are reflected by polished chromium.Compounds of Chromium
Chromium(III) compounds include chromium(III) nitrate, chromium(III) acetate, and chromium(III) oxide, among many more. Chromium(III) can be produced by reducing chromium(VI) by cytochrome c7, or it can be obtained by dissolving elemental chromium in acids like sulfuric or hydrochloric acid.Also Check - Bond Order Formula
Octahedral compounds frequently form with chromium(III). The dark green complex [CrC l 2 ( H 2 O ) 4 ]Cl is the chromium(III) chloride hydrate that is readily accessible. The pale green [CrCl( H 2 O ) 5 ]C l 2 and the violet [Cr( H 2 O ) 6 ]Cl ) 3 are closely related substances. Anhydrous violet chromium(III) chloride dissolves in water and, over time, the chloride in the inner coordination sphere is replaced by water, turning the violet solution green.Download PDF Chromium III Sulfate Formula
Chromium III Sulfate Formula
The Chromium III sulfate general formula is C r 2 (S O 4 ) 3 . An inorganic substance called chromium III sulphate has a chromium to sulphate ratio of 2:3. C r 2 (S O 4 ) 3 is the formula for chromium III sulphate. It is a blue-grey solid that is also water-soluble. As it warms up, the colour changes from blue to green. Here, let's study the formula for chromium III sulphate.Also Check - Charles Law Formula
The inorganic compounds known as chromium (III) sulphate typically have the formula C r 2 (S O 4 ) 3 .x H 2 O , where x is a number between 0 and 18. Furthermore, vague but crucial to business "basic chromium sulphates" are well recognised. These salts often contain particles that are soluble in water and are either violet or green in colour. We frequently use it for tanning leather. [caption id="attachment_14953" align="alignnone" width="300"]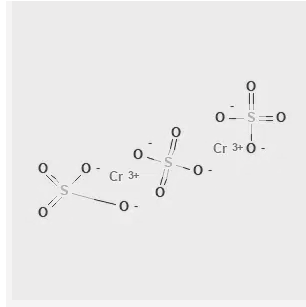 Chromium III Sulfate Structure[/caption]
Chromium III Sulfate Structure[/caption]
Types of Chromium III Sulfate
The following are the three well-characterized chromium III sulphates: When a reducing agent is added to the violet-coloured solid anhydrous chromium III sulphate, C r 2 (S O 4 ) 3 , it dissolves inside the water and produces chromium(II) sulphates. The violet-coloured hydrated chromium III sulphate, Cr2(SO4)3•18H2O, quickly dissolves in water to form the metal aqua complex, [Cr( H 2 O ) 6 ] 3+ . The formula for this chemical may be written as [Cr( H 2 O ) 6 ] 2 (S O 2 ) 3 •6 H 2 O . In this formula, 6 of the 18 water molecules are crystallisation water.Also Check - Combined Gas Law Formula
The hydrated form of chromium III sulphate, C r 2 (S O 2 ) 3 •15( H 2 O) , has a green colour and is easily dissolved in water. By raising the temperature of the 18-hydrate substance over 70 °C, we may quickly get it. Anhydrous sulphate can also be produced with further heating. It is also known that several additional chromium (VI) sulphates have hydroxide or oxide ligands. The basic form of chromium sulphate, which is assumed to be [C r 2 ( H 2 O ) 6 (OH ) 4 ]S O 4 , is the most significant and valuable in terms of commerce. It happens as a result of the hexahydrates' partial neutralisation. The other chromium III hydroxides, however, have been documented.Chromium III Sulfate Production
The Cr(III) wastes that we produce from the chromate oxidation of a variety of different organic compounds are the most beneficial and well-liked source of chromium (III) sulphate. Anthracene and phenol are subjected to chromic acid treatment in order to make anthraquinone and quinone on a big scale. The co-product generated chromium (III) oxide, is easily extracted into sulfuric acid. The hydrated salt stated above is easily accessible through the evaporation of these acidic solutions. Furthermore, the extraction of numerous other chromium compounds can also result in the production of hydrated salts of chromium sulphate, albeit impurely; however, the extraction of chromite ore with sulfuric acid in the presence of some chromate yields solutions of chromium (III) sulphate contaminated with other metal ions. Similar to this, the chromium sulphate and ferrous sulphate are produced when the chrome alloys dissolve.Chromium III Sulfate Formula FAQs
What is the purpose of chromium III sulphate?
Chromic Sulphate is a powder that is peach in colour and odourless. It is utilised in ceramics, leather tanning, chrome plating, green paints, inks, and textile dyes, as well as the photography sector.
Chromium III Sulfate has what kind of a structure?
Chromium III Sulfate's chemical formula is Cr2(SO4)3. It is an ionic combination made up of the metal chromium (Cr) with the Cr3+ cation and the SO42- anion of sulphate.
Where is the valency of ions in Chromium III Sulfate?
Sulphate has a valence of 2, whereas Chromium III has a valence of 3, therefore to balance the six valence ties, we will require 2 chromium and 3 sulphate ions.
Does chromium benefit plants?
The high toxicity of chromium, a non-essential metal for plants, impacts the germination of plant seeds as well as the growth and development of roots and shoots.
Does Chromium III Sulfate dissolve in water?
An inorganic compound with a chromium to sulphate ratio of 2: 3, chromium (sulphate III), is used in electronics. Chromic (III) sulphate is represented by the formula Cr2(SO4)3. It also dissolves in water and is a solid blue-grey colour.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









