
In order to understand the definition of salt weak acid and strong base formula , one must learn about acids, bases and salts. A base and an acid are present in the salt ionic substance. It makes up most of the minerals in salt water, which contains vast amounts. Salt is necessary for animal existence, and saltiness is one of the fundamental tastes for humans. In the neutralisation process between acids and bases, salt is a kind of ionic molecule that includes water and a cation other than H+ and an anion other than O H - , CuC l 2 , NaCl , etc.
Water +Salt+ Acid=Base One of the most well-known salts is sodium chloride. Due to its frequent daily use, practically everyone is familiar with one salt.Difference Between Strong Acids And Bases
Strong acids and bases ionise when water dissolves, while weak acids and bases only partially ionise in their solutions. The following lists a few typical weak acids and bases. We frequently run with weak acids and bases in academic difficulties and day-to-day living, making them fairly regular.Also Check - Acids and Bases Formula: Types of Reaction
A phenomenon of chemical equilibrium is the ionisation of weak acids and bases. The concepts of equilibrium are crucial for a knowledge of weak acid and weak base equilibria. You are undoubtedly already aware that weak bases are weak bases, and weak bases are conjugate bases of weak acids. This difference leads to the chemistry definition of salt of weak acid and strong base.Characteristics Of Strong And Weak Acids And Bases
Strong acids are less prevalent than weak acids. They can be found in everyday items like vinegar (acetic acid) and lemon juice (citric acid). Simple arrows pointing left to right represent the reaction of a strong acid ionising in water. On the other hand, a weak acid ionising in water has a double arrow reaction arrow, suggesting that both the forward and reverse reactions take place at equilibrium. At equilibrium, the aqueous solution contains the weak acid, its conjugate base, and the hydrogen ion. A chemical bond's polarity or electron distribution determines whether or not an acid entirely ionises in water. When two atoms in a bond have electronegativity values that are almost the same, the electrons are equally distributed and spend almost equal amounts of time on each atom (a nonpolar bond). However, when there is a considerable difference in electronegativity between the atoms, there is a charge separation, which causes electrons to be pulled to one atom (polar bond or ionic bond) more than the other.Also Check - PH of Weak Acid Formula
Hydrogen atoms acquire a tiny positive charge when joined to an electronegative element. Hydrogen is more readily ionised and produces an acidic molecule if the electron density surrounding it is lower. Weak acids can occur when the hydrogen atom and the other atom in the connection are not sufficiently polar because the hydrogen ion is difficult to remove. The atom's size coupled to the hydrogen atom also influences an acid's effectiveness. The link between the two atoms weakens as the atom grows larger. As a result, the hydrogen bond is more easily broken, and the acid is enhanced. We have learned that at temperatures below zero, water turns to ice. Water's freezing point drops below zero when solutes or contaminants like salt and sugar are present. However, when solutes or impurities like salt and sugar are present in water, its freezing point becomes lower than zero. This implies that the solution of salt and water remains liquid even as the temperature approaches zero. This phenomenon is termed a lowering of the freezing point or depression in the freezing point.Chemistry Definition of Salt
The name "salt" comes from the Latin word "sal," which implies salt. It was formerly a valuable commodity and has been used for trade. The English term "salary" has its roots in salt. Salt has long been used to flavour and preserve food. It has also produced ceramics, soap, chlorine, dyes, and bleaches. It is widely used in the chemical industry nowadays.Also Check - Aluminium Nitrate Formula
It commonly appears as kosher salt, rock salt, sea salt, or free-flowing table salt at or in the kitchen. Fast food and frozen chicken are only two examples of foods with high sodium or salt content.Chemistry Definition Of Salt Of Weak Acid and Strong Base
A salt that includes the weak acid's conjugate base is created when a strong base neutralises a weak acid. This is typical; this conjugate base is weak. This is the chemistry Definition Of Salt Of Weak Acid and Strong Base. For instance, sodium acetate, NaC H 3 C O 2, is a salt created when the strong base, sodium hydroxide, reacts with the weak acid acetic acid. This salt is a solution of sodium ions and acetate ions. The acidity of the solution is unaffected by the sodium ion. The conjugate base of acetic acid, the acetate ion, interacts with water and raises the concentration of the hydroxide ion. C H 3 C O 2 (aq)+ H 2 O(l) ⇌ C H 3 C O 2 H(aq)+OH(aq) [caption id="attachment_19344" align="alignnone" width="386"]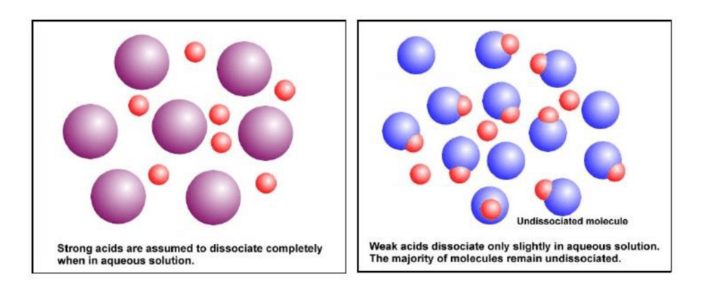 Chemistry definition of salt of weak acid and strong base[/caption]
Chemistry definition of salt of weak acid and strong base[/caption]
Chemistry Definition of Salt Weak Acid and Strong Base FAQs
What distinguishes a strong acid from a weak base?
Is one per cent ionised a weak acid?
If a powerful acid does not completely dissociate, what is it?
What does salt mean in acid, base, and salt?
What exactly does it mean to have a strong acid and a strong base in salt form?










