
Cadmium Sulfate Formula: Cadmium, symbolized as Cd with an atomic number of 48, is a malleable and ductile metal known for its shiny silver-white appearance. This toxic heavy metallic element is widely distributed in the Earth's crust. Notably, it finds application in nuclear reactors where it serves as an absorber to regulate neutron reaction rates, and it was initially identified by Friedrich Stromeyer.
Sulfur, represented by the symbol S and with an atomic number of 16, exhibits the electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 4 and is classified within the oxygen group. This versatile nonmetal is abundant and exhibits multiple valence states, serving in various roles, including the production of sulfuric acid, batteries, and cleaning agents. It has limited thermal and electrical conductivity.
Oxygen, symbolized as O and possessing an atomic number of 8, demonstrates an electron configuration of 1s 2 2s 2 2p 4 , and it belongs to the chalcogen group in the periodic table. Oxygen holds a pivotal role in respiration and finds extensive use in industrial processes and water treatment.
Cadmium Sulfate Formula
Cadmium Sulfate formula is CdSO 4 . It is an inorganic compound that presents as a soluble white crystalline solid when in contact with water. This compound is extensively used in the process of electroplating cadmium onto electronic circuitry. It occurs naturally in the Earth's crust and is typically found as a mineral combined with other elements.
When cadmium sulfate heated, cadmium sulfate produces hazardous cadmium oxide fumes. Notably, it ranks as one of the foremost environmental pollutants, following only mercury and lead. This compound consists of one cadmium atom surrounded by one sulfur atom and four oxygen atoms. Notably, it is acknowledged as a carcinogen and associated with an elevated likelihood of developing lung cancer.
Cadmium Sulfate Formula Structure
Cadmium Sulfate formula is CdSO 4 . It is an inorganic compound consisting of a cadmium cation and a sulfate anion. Within the sulfate ion, sulfur serves as the central atom, and it is surrounded by four oxygen atoms. The Lewis structure of Cadmium Sulfate is illustrated in the diagram provided, and the compound is interconnected through ionic bonds.
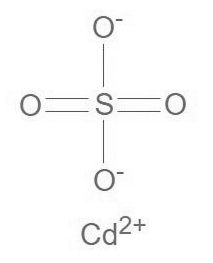
Cadmium Sulfate Preparation
Cadmium Sulfate can be formed through provided reactions. below.
When sulfuric acid reacts with cadmium. It produces cadmium sulfate, water, and sulfur dioxide.
2 H 2 SO 4 + Cd → 2 H 2 O + SO 2 + CdSO 4
Cadmium reacts with sodium persulfate to yield cadmium sulfate and sodium sulfate.
Cd + Na 2 S 2 O 8 → Na 2 SO 4 + CdSO 4
The combination of sulfuric acid and cadmium hydroxide results in the formation of cadmium sulfate and water.
H 2 SO 4 + Cd(OH) 2 → 2 H 2 O + CdSO 4
Cadmium sulfate can also be obtained by reacting sulfuric acid with cadmium oxide, leading to the formation of water and cadmium sulfate.
H 2 SO 4 + CdO → H 2 O + CdSO 4
Cadmium Sulfate Formula Physical Properties
Cadmium Sulfate has a molecular weight of 208.47 g/mol.
Its density measures 4.691 g/cm3.
The compound has a melting point of 1000°C.
When heated to its boiling point, it undergoes decomposition, resulting in the formation of basic sulfate and then oxide compounds.
Cadmium Sulfate Formula Chemical Properties
The Cadmium Sulfate Formula is CdSO 4 .
Cadmium Sulfate exhibits various chemical reactions, such as:
Reacting with Sodium Hydroxide to yield sodium sulfate and cadmium hydroxide:
2 NaOH + CdSO 4 → Na 2 SO 4 + Cd(OH) 2
Reacting with Zinc to form Zinc Sulfate and cadmium:
Zn + CdSO 4 → ZnSO 4 + Cd
Reacting with Chromium to produce Chromous Sulfate and cadmium:
Cr + CdSO 4 → Cd + CrSO 4
Reacting with Ammonium Hydroxide to generate Cadmium hydroxide and ammonium tetraoxosulfate:
2 NH 4 OH + CdSO 4 → Cd(OH) 2 + (NH4) 2 SO 4
Reacting with Sodium Sulfide to create Cadmium Sulfide and sodium sulfate:
Na 2 S + CdSO 4 → Na 2 SO 4 + CdS
Health Effects of Cadmium Sulfate
Cadmium sulfate is known to be toxic to human beings. Exposure to this substance can lead to various health concerns, including:
- Irritation of the eyes and skin.
- Inhalation of cadmium sulfate can cause irritation to the nose and throat.
- Adverse effects on human kidneys and lungs.
- Classified as a carcinogenic substance to humans, increasing the risk of cancer.
- Symptoms of exposure may include nausea, diarrhea, vomiting, and abdominal pain
Uses of Cadmium Sulfate
Cadmium Sulfate is utilized in several applications, including:
- Production of solar cells.
- Electroplating processes.
- Use as a pigment in fluorescent screens.
- Used as an electrolyte in the Weston standard cell.
- Application in chemical bath deposition for photoresistors.
- Use as a chemical intermediate in various chemical processes.
| Related Links | |
| Sodium Acetate Formula | Carbonous Acid Formula |
| Cholestrol Formula | Chlorine Gas Formula |
Cadmium Sulfate Formula FAQs
What is Cadmium Sulfate chemical formula?
What is cadmium's atomic number?
Why is cadmium used in nuclear reactors?
What is the primary sulfur source for Cadmium Sulfate production?
Is Cadmium Sulfate soluble in water?










