
Calcium Acetate Formula: Calcium acetate formula is Ca(C 2 H 3 O 2 ) 2 . This compound holds a unique place in the world of chemistry. It's a calcium salt of acetic acid known for its various applications, both in the laboratory and in everyday life. This compound, also referred to as calcium ethanoate or acetate of lime, is primarily valued for its role in binding phosphate and its use as a food additive, among other purposes.
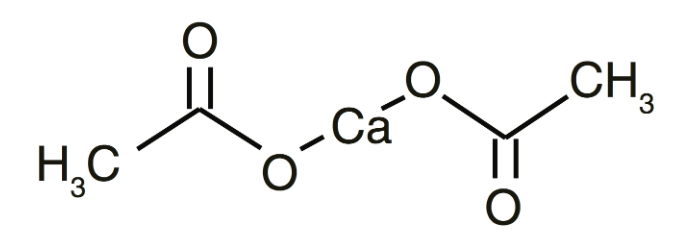
Calcium Acetate Formula Structure
The Calcium Acetate formula is C 4 H 6 CaO 4 . It exists as a colorless to white crystalline solid and hold a faint acetic acid odor. This compound readily dissolves in both water and alcohol, while remaining insoluble in benzene and acetone.
Calcium Acetate Formula Physical Properties
Properties of Calcium Acetate Formula
- Chemical Formula: Calcium Acetate formula is C 4 H 6 CaO 4.
- Molecular Weight: 158.166 g/mol
- Density: 1.509 g/cm3
- Refractive Index: 1.55
- Melting Point: 160°C
Calcium Acetate Formula Chemical Properties
Calcium acetate exhibits chelating properties, enabling it to engage in chelation, a process where it forms additional coordination bonds. Due to the presence of a metal atom, calcium, capable of binding to other chemical ions, calcium acetate can perform this function effectively.
Furthermore, the calcium atoms within this compound play a crucial role in the regulation of blood phosphate levels. This is achieved through the reaction of calcium acetate, leading to the formation of insoluble phosphate, simplifying its management.
Production Calcium Acetate
Calcium acetate can be produced through simple chemical reactions. It can be obtained by soaking calcium carbonate, which can be found in various natural sources such as eggshells, limestone, or marble, in vinegar. The reaction can be represented as follows:
CaCO 3 (s) + 2CH 3 COOH(aq) → Ca(CH 3 COO) 2 (aq) + H 2 O(l) + CO2(g)
This straightforward method of production suggests that calcium acetate might have been observed in nature historically, as the necessary ingredients were readily available.
Another method of production involves reacting hydrated lime (Ca(OH) 2 ) with acetic acid (CH 3 COOH):
Ca(OH) 2 (s) + 2CH 3 COOH(aq) → Ca(CH 3 COO)2(aq) + 2H 2 O(l)
Calcium acetate is commonly found in the monohydrate form, Ca(CH 3 COO) 2 •H 2 O, as the anhydrous form tends to be highly hygroscopic, absorbing moisture readily.
Uses of Calcium Acetate
Phosphate Binding in Kidney Disease: Calcium acetate plays a vital role in the medical field, especially concerning patients with kidney disease. In such instances, elevated blood phosphate levels can lead to a condition called hyperphosphatemia, which can result in a range of bone-related issues. Calcium acetate is used to effectively bind dietary phosphate, thus reducing blood phosphate levels and mitigating the related health concerns.
Food Additive: Calcium acetate is used as a food additive, where it serves as a stabilizer, buffer, and sequestrant, often given the E number E263. It finds widespread use in the food industry, especially in candy products.
Tofu Production: Traditionally, tofu, a popular soy-based food, is obtained by coagulating soy milk using calcium sulfate. However, calcium acetate has emerged as a more convenient alternative. It is soluble and requires less skill and a smaller quantity for the coagulation process.
Historical Use in Acetone Synthesis: Before the development of the cumene process, calcium acetate was a common starting material for the synthesis of acetone. The reaction involves the decomposition of calcium acetate into calcium carbonate and acetone.
Ca(CH 3 COO) 2 → CaCO 3 (s) + (CH 3 ) 2 CO
California Snowballs: Chemistry teachers often use calcium acetate to create a fun experiment known as "California Snowballs." By preparing a saturated solution of calcium acetate in alcohol, they can form a semisolid, flammable gel that resembles snowballs. This experiment is an engaging way to demonstrate chemical concepts in a classroom setting.
Natural Occurrence of Calcium Acetate
While calcium acetate is a well-known compound, it has not been found in a pure, naturally occurring mineral form. Calclacite, which is listed as calcium acetate chloride pentahydrate, is considered a known mineral. However, its genesis is likely anthropogenic, meaning it is generated by human activity rather than naturally occurring, and it may not qualify as a true mineral in a geological sense.
Calcium acetate is a fascinating compound with diverse applications, ranging from its use in the treatment of kidney disease to its role in the food industry and even in entertaining chemistry experiments. Its simple production methods and historical relevance make it a noteworthy compound in the world of chemistry and everyday life.
| Related Links | |
| Inorganic Compound Formula | Aluminium chloride Formula |
| Electronic configuration of copper Formula | Aluminium fluoride Formula |
Calcium Acetate Formula FAQs
What is the chemical formula of calcium acetate?
What is the molecular weight of calcium acetate?
What is the density of calcium acetate?
What is the refractive index of calcium acetate?
At what temperature does calcium acetate melt?










