
Chloroplatinic Acid Formula is H 2 PtCl 6 . Platinum is an element on the periodic table with the atomic number 78 and symbol Pt, derives its name from the Spanish word "Platina," which translates to silver. This transition metal exhibits notable characteristics, including malleability, ductility, high density, and a white-silver appearance. It possesses six naturally occurring isotopes and is one of the rarest elements in the Earth's crust, thus holding significant value. Notably, platinum is highly unreactive.
Chlorine, belonging to the halogen group on the periodic table, bears the atomic number 17 and the symbol Cl. This element typically appears as a yellow-green gas at room temperature and exhibits extreme reactivity, functioning as a potent oxidizing agent. With the highest electron affinity across the periodic table, chlorine is predominantly found in ionic form within the Earth's crust due to its high electronegativity.
Chloroplatinic Acid Formula
Chloroplatinic acid, also known as hexachloroplatinic, is an inorganic compound that presents as a red solid. This substance holds significant importance in commercial applications, being one of the key forms of platinum utilized. It is essentially the hydronium salt of the hexachloroplatinate anion. Chloroplatinic acid exhibits high hygroscopicity, indicating its strong water-absorbing capacity. The Chloroplatinic Acid Formula is [H 3 O] 2 [PtCl 6 ](H 2 O) x , although for convenience, it can also be expressed as H 2 PtCl 6 . This compound readily dissolves in water, resulting in a mildly acidic solution. Its uses encompass the production of indelible ink and its involvement in the electroplating process.Chloroplatinic Acid Formula Structure
The structure of Chloroplatinic acid, also known as hexachloroplatinic, is noteworthy. It is an inorganic compound with the IUPAC name "tetrachloroplatinum; hydrate; dihydrochloride." The complex displays an octahedral geometry, giving it a distinct shape. In its natural state, Chloroplatinic acid presents as a red solid.
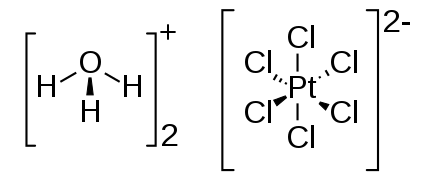 Chloroplatinic Acid Formula is [H
3
O]
2
[PtCl
6
](H
2
O)
x
, although for simplicity, it can also be denoted as H
2
PtCl
6
. This compound has a molecular weight of 427 atomic mass units (amu). The bond between [H
3
O and [PtCl
6
] is characterized as a coordination covalent bond, which is a fundamental feature of its structural composition.
Chloroplatinic Acid Formula is [H
3
O]
2
[PtCl
6
](H
2
O)
x
, although for simplicity, it can also be denoted as H
2
PtCl
6
. This compound has a molecular weight of 427 atomic mass units (amu). The bond between [H
3
O and [PtCl
6
] is characterized as a coordination covalent bond, which is a fundamental feature of its structural composition.
Chloroplatinic Acid Preparation
Chloroplatinic acid can be prepared using various methods, including:
Dissolving Platinum in Aqua Regia: One of the most widely used methods involves dissolving platinum in aqua regia, which is a mixture of nitric acid and hydrochloric acid in a 1:3 ratio. The chemical reaction is as follows:
Pt + 4 HNO 3 + 6 H 2 O → H 2 PtCl 6 + 4 NO 2 + 4 H 2 OExposing Platinum Particles to Chlorine Gas: Chloroplatinic acid can also be produced by exposing an aqueous suspension of platinum particles to chlorine gas.
Electrolysis: Another method involves the use of electrolysis to generate Chloroplatinic acid.
These methods are used in the synthesis of Chloroplatinic acid for various industrial applications.
Chloroplatinic Acid Formula Physical Properties
- Chloroplatinic acid has a molecular weight of 427 amu.
- In its natural state, Chloroplatinic acid appears as a reddish-brown solid.
- It exhibits solubility in water, giving rise to a mildly acidic solution.
- While it is inflammable, it is challenging to ignite.
- Chloroplatinic acid is characterized by an acidic odor.
Chloroplatinic Acid Formula Chemical Properties
Upon heating, Chloroplatinic acid undergoes decomposition to form Platinum(IV) Chloride:
(H 3 O) 2 PtCl 6 ·xH 2 O → PtCl 4 + 2 HCl + (x + 2) H 2 O- It has a melting point of 60 degrees Celsius.
- Chloroplatinic acid decomposes when subjected to heat.
- The density of Chloroplatinic acid is measured at 1.05 g/ml.
- When combined with water, it results in an acidic solution with a pH value below 7.
Health Hazards of Chloroplatinic Acid
- Chloroplatinic Acid is known to trigger allergies and asthma in individuals exposed to it.
- Prolonged exposure to Chloroplatinic Acid over an extended period can result in permanent lung damage.
- Ingesting Chloroplatinic Acid can be lethal.
- Contact with the eyes can lead to irritation and redness.
Uses of Chloroplatinic Acid
Chloroplatinic acid plays a crucial role in the quantitative analysis of potassium, aiding in the determination of potassium levels. It is favored for this purpose over Sodium Cobaltinitrite due to its advantages.
It serves as a significant source for the commercial production of platinum. The processing of Chloroplatinic acid is instrumental in the widespread production of platinum.
Chloroplatinic acid is used in the purification of platinum. When ammonium salts are treated with Chloroplatinic acid, they transform into elemental platinum, forming ammonium hexachloroplatinate.
This compound is commonly used as a catalyst in various chemical reactions. For example, it acts as a catalyst in the conversion of hydrosilanes to olefins.
| Related Links | |
| Potassium Chlorate Formula | Potassium Bromate Formula |
| Gold Formula | Phosphate Formula |
Chloroplatinic Acid Formula FAQs
What is the chemical formula of Chloroplatinic Acid?
What is Chloroplatinic Acid's appearance at room temperature?
Is Chloroplatinic Acid soluble in water?
What are some common methods for preparing Chloroplatinic Acid?
What is the structural geometry of Chloroplatinic Acid?










