
Potassium Bromate Formula: Potassium is a chemical element represented by symbol ‘K’, its atomic number is 19 and electronics configurations iis 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 . It belongs to the first group and the fourth period of the periodic table. Potassium is commonly used to address low potassium levels and occurs naturally in minerals and salt.
Bromine, on the other hand, is another chemical element denoted by the symbol Br, possessing an atomic number of 35 and an electron configuration of [Ar] 3d 10 4s 2 4p 5 . It falls into the 17th group and the fourth period of the periodic table as a P-Block element.
Oxygen, symbolized as "O," holds an atomic number of 8 and has an electron configuration of 1s 2 2s 2 2p 4 . It belongs to the chalcogen group on the periodic table. Oxygen plays a vital role in respiration, being a colorless, odorless, diatomic gas that constitutes 21% of the atmosphere. Its primary application is in the controlled oxidation of chemicals.
Potassium Bromate Formula
Now, when we talk about Potassium Bromate formula (KBrO 3 ), it is a commonly used form of bromine with a chemical formula of KBrO 3 . It appears as white crystals or powder and is a strong oxidizing agent.
Potassium bromate is an ionic compound, there are two ions is K + and BrO3 – , and it's classified as an inorganic compound. Additionally, it has a high melting point and is water-soluble but moderately toxic, causing symptoms such as vomiting, diarrhea, respiratory stimulation, and renal injury.
The compound KBrO 3 is named "Potassium Bromate," consisting of potassium, bromine, and oxygen atoms. It is commonly used as a flour improver and oxidizing agent in baking.
Potassium Bromate Formula Structure
The chemical structure of potassium bromate (KBrO 3 ) combined with potassium (K) cation, a bromine (Br) anion, and three oxygen (O) atoms. These elements are bonded together to form the compound.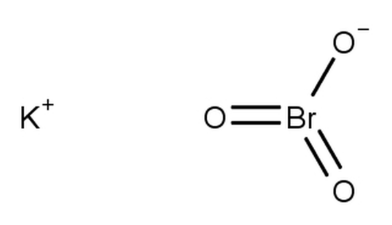
Potassium Bromate Formula Charge
The potassium bromate formula is KBrO 3 . In this compound:K represents potassium, which has a charge of +1.
Br represents bromine, which has a charge of -1.
O represents oxygen, which also has a charge of -2.
The overall charge of potassium bromate (KBrO 3 ) is neutral because the charges of the individual elements balance each other out.Potassium Bromate Formula Physical Properties
Below are physical properties of Potassium Bromate:
- Molar mass: 167.00 g/mol
- Density: 3.27 g/cm3
- Boiling point: 370°C
- Melting point: 350°C
Potassium Bromate Formula Chemical Properties
Below are chemical properties of Potassium Bromate:
- Chemical formula: KBrO 3
- Appearance: Odorless white crystalline powder
- Solubility: Slightly soluble in alcohol, insoluble in acetone and ethanol
- Reaction: The reaction involving potassium bromate and sodium bromate in the presence of a dilute acid solution leads to the generation of bromine. The corresponding chemical equation for this process is as follows.
Potassium Bromate Harmful Effect
Potassium bromate poses significant health hazards. Ingesting it may result in symptoms such as nausea, vomiting, diarrhea, respiratory difficulties, decreased body temperature, methemoglobinemia, and potential kidney damage. Furthermore, in certain instances, its use in toothpaste has been associated with gum inflammation and bleeding.
Potassium Bromate Uses
Potassium Bromate serves various purposes, such as:
- Strengthening dough and improving its elasticity as an oxidizer.
- Ensuring uniform and whitened bread when used in baking.
- Enhancing the quality of flour as a flour improver.
- Playing a role in the production of malt barley.
- Serving as a source of bromine.
- Being included in toothpaste as an antiseptic and astringent.
| Related Links | |
| Chromium III Chloride Formula | Citric Acid Formula |
| Cinnamic Acid Formula | Trichloroacetic Acid Formula |
What is the chemical formula for Potassium Bromate?
What are the key elements in Potassium Bromate?
Is Potassium Bromate an inorganic or organic compound?
What are some physical properties of Potassium Bromate?
Can Potassium Bromate dissolve in water?










