
Gold Formula: Gold is a chemical element with the symbol Au (from the Latin "aurum") and atomic number 79. Gold appears as nuggets or granules in various geological formations such as rocks, veins, and alluvial deposits. It also exists in a natural blend with silver, forming an alloy with other metals like copper and palladium, and can be found as mineral inclusions like pyrite.
Gold, denoted by the symbol Au with the atomic number 79, is among the naturally occurring elements with a relatively high atomic number. In its purest state, it boasts a brilliant, somewhat bright yellow color and possesses qualities of being dense, soft, malleable, and ductile.
In Chemistry gold is classified as both a transition metal and a member of group 11. It ranks among the least reactive elements in the periodic table and typically maintains a solid state under standard conditions.
The Presence of Gold
Gold is a rare metal in rocks that come from volcanoes. In the Earth's rocky layer, we find only a tiny bit of gold, around 0.005 parts for every million parts. Unlike some other elements, like tellurium, selenium, and bismuth, gold is usually on its own without mixing with other stuff. And when we look at its different forms, there's one kind called Gold-197 which is the element of naturally occurring isotopes.
Gold is commonly discovered in association with deposits of copper and lead. Although the quantities found are typically quite small, it can be easily recovered as a byproduct during the refinement of these base metals. Large masses of gold-rich rock, substantial enough to be called ores, are rare to come across. There are two main categories of gold deposits: hydrothermal veins, where it is often found alongside quartz and pyrite, and placer deposits, both consolidated and unconsolidated, which are formed through the weathering of rocks containing gold.
Gold Formula Structure
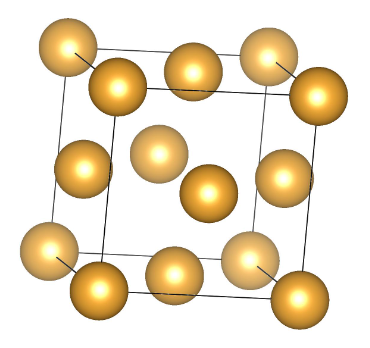
The structure of gold, like many metals, is a crystalline structure. Gold has a face-centered cubic (fcc) crystal structure. In a face-centered cubic structure, atoms are arranged in a cube, and there is an atom at each corner of the cube and one in the center of each face of the cube. This arrangement results in a highly symmetric and closely packed structure.
In the case of gold:
Each corner of the cube is occupied by a gold atom.
There is one gold atom at the center of each face of the cube.
This arrangement gives gold its characteristic metallic properties, including its malleability and ductility, which allow it to be easily shaped and formed without breaking. The fcc crystal structure also contributes to gold's high reflectivity and lustrous appearance.
Gold's face-centered cubic structure is a key factor in its unique combination of physical and chemical properties, making it highly valued for a wide range of applications, from jewelry to electronics and beyond. Below are Gold formula structure.
Gold Formula Properties
- Chemical Formula: Au
- Atomic Number (Z): 79
- Group, Period, and Block: Group 11, 6th period, d-block
- Electronic Configuration: [Xe] 4f 14 5d 10 6s 1
- Electrons per Shell: 2, 8, 18, 32, 18, 1
- Density: 19.30 grams per cubic centimeter (g/cm³)
- Boiling Point: 3243 Kelvin (K)
- Melting Point: 1337.33 K
- Crystal Structure: Face-centered cubic
Gold is a remarkably rare and precious metal that doesn't rust or pose any toxicity concerns. It's highly resistant to corrosion and can be found in alluvial and vein deposits.
While copper and silver are known for their excellent heat and electricity conduction, gold connections outshine them due to their resistance to tarnishing. This means that gold connections remain effective for a longer period.
Gold is incredibly malleable, capable of being drawn out into extremely thin wires. Just one ounce of gold can be stretched into a wire measuring 80 kilometers long, with a thickness as fine as five microns (or five millionths of a meter), having a diameter of 0.20 millimeters.
Furthermore, gold is highly reflective of both heat and light, making it an ideal choice for coating the visors of astronauts' space helmets. This thin, partly transparent layer of gold reduces glare and heat from the sun while allowing astronauts to see through it.
Gold's aesthetic beauty is also well-regarded. It's often alloyed with other metals in jewelry to enhance its strength, and the term "carat" indicates the amount of gold present (with 24 carats representing pure gold). Gold is cherished by jewelers and metalsmiths for its versatility, as it can be embossed, hammered, cast, stretched, or twisted. Its malleability allows it to be flattened into incredibly thin sheets, with just one ounce of gold capable of covering more than 9 square meters.
Uses of Gold
Gold serves various purposes in different industries. It is used in the creation of bullion, jewelry, glass, and electronics. Jewelry production accounts for about 75% of the total gold output, with the metal's color changing based on the alloys it's combined with. In the glass industry, colloidal gold lends its red or purple hue, while metallic gold is applied as a thin film on large building windows to reflect the sun's heat. In electronics, gold electroplating safeguards copper components and improves solderability.
Gold is also used in medicine, like a special kind called Au-198, which has some radiation. Doctors use it to help treat cancer. They also make gold thread for embroidery. Gold can be a thin layer on big building windows to bounce the sun's rays. Gold's many abilities and special traits make it super useful in many areas.
| Related Links | |
| Hydrochloric Acid Formula | Hydrazine Formula |
| Phosphate Formula | Helium Formula |
Gold Formula FAQs
What Symbol represents gold in chemistry?
What is the atomic number of Au?
What's the shape of gold's atomic structure?
What is the boiling point and melting point of gold?










