
Oxalic Acid Formula is C2H2O4. It is also referred as ethanedioic acid; oxalic acid is a potent dicarboxylic acid in terms of both its nature and structure. It can be found naturally in plants and vegetables and is also produced within the body through the metabolic breakdown of ascorbic acid or glyoxylic acid. Due to its versatile properties, oxalic acid has numerous applications, particularly in the cleaning industry for laundry, bleaching, and dyeing tasks. Oxalic acid is a well-known organic compound with a distinct chemical formula.
Oxalic Acid Formula
The chemical formula for oxalic acid is (COOH)_2 or C2H2O4. This formula reflects the molecular composition of the compound, which consists of two carbon atoms (C), two oxygen atoms (O), and four hydrogen atoms (H). Carboxyl groups (COOH) in its structure is a defining characteristic of oxalic acid.
Also Read: Galactose Formula
What is Oxalic Acid?
Oxalic acid is the simplest dicarboxylic acid also known as Oxiric Acid. It has the IUPAC name Ethanedioic Acid and a HOOC- COOH molecular formula.
In 1745, Dutch scientist Herman Boerhaave extracted oxalic acid salts from wood sorrel. In 1773, François Pierre Savary isolated oxalic acids from these salts. In 1776, synthetic oxalic acid was prepared in the laboratory.
Globally, 120,000 tonnes of oxalic acid are produced every year.
Also Read: Glucose Chemical Formula
Oxalic Acid Formula Structure
The structure of oxalic acid is relatively simple but essential to understand its properties. Oxalic acid is a dicarboxylic acid containing two carboxyl (-COOH) functional groups. These carboxyl groups are attached to the carbon atoms in the molecule. The structure can be depicted as follows:
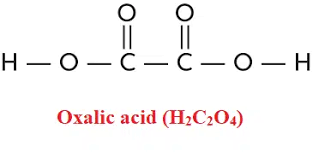
Each of the two carbon atoms in the structure is bonded to an oxygen atom and a hydroxyl (-OH) group in addition to the carboxyl groups. The oxygen atoms form double bonds with the carbon atoms, giving the molecule a planar, symmetrical structure.
Oxalic Acid Formula N Factor
The n factor (known as the normality factor) of oxalic acid is related to its ability to donate or accept protons (H+ ions) in a chemical reaction. Oxalic acid has two carboxyl groups; each can donate two protons. Therefore, the n-factor of oxalic acid is 4.
Oxalic Acid Formula Molar Mass
The molar mass of a substance is the mass of one mole of that substance. To calculate the molar mass of oxalic acid, we sum the atomic masses of all the atoms in the molecular formula. The molar mass of oxalic acid (C2H2O4) can be calculated as follows:
- 2 carbon atoms (C) with an atomic mass of approximately 12.01 g/mol each.
- 2 hydrogen atoms (H) with an atomic mass of approximately 1.01 g/mol each.
- 4 oxygen atoms (O) with an atomic mass of approximately 16.00 g/mol each.
Molar Mass of Oxalic Acid = (2 * 12.01 g/mol) + (2 * 1.01 g/mol) + (4 * 16.00 g/mol) ≈ 90.03 g/mol
Also Read: Glutaric Acid Formula
Oxalic Acid Formula Equivalent Weight
The equivalent weight of oxalic acid is a measure of the amount of the substance that can either gain or lose one equivalent of protons (H+ ions) in a chemical reaction. Since the n factor of oxalic acid is 4, the equivalent weight is equal to the molar mass divided by 4.
Equivalent Weight of Oxalic Acid = Molar Mass of Oxalic Acid / n factor
Equivalent Weight of Oxalic Acid = 90.03 g/mol / 4 ≈ 22.51 g/equiv
Oxalic Acid Formula Mass
The formula mass of oxalic acid is simply the sum of the atomic masses of the atoms in its formula, without considering the Avogadro's number (which is used for molar mass calculations). The formula mass of oxalic acid is also approximately 90.03 atomic mass units (amu).
Oxalic acid is a unique organic compound with a distinctive formula, structure, and properties. Understanding its formula, structure, n factor, molar mass, equivalent weight, and formula mass is essential for various chemical and analytical applications.
| Related Links | |
| Calcium Bromide Formula | Citric Acid Formula |
| Calcium Iodide Formula | Condensed Structural Formula |
Oxalic Acid Formula FAQs
Is COOH an oxalic acid?
What is the other name of oxalic acid?
Is the molar mass of oxalic acid 90 or 126?
What is the formula for oxalic acid?
What is COOH called?










