
Galactose Formula is C 6 H 12 O 6 . It's a type of sugar called a monosaccharide, and it's closely related to glucose. One interesting thing about galactose is its ability to form hydrogen bonds. It can act as a hydrogen bond donor at 5 different sites and a hydrogen bond acceptor at 6 sites.
The Galactose Formula, is a monosaccharide with about the same sweetness level as glucose and as sweet as sucrose. This glucose C-4 epimer was first discovered by E. O. Erdmann in 1855 through the hydrolysis of lactose, which produced galactose (although it wasn't recognized then). Galactose Formula can exist in both open-chain and cyclic forms. It can be found in various sources such as dairy products, avocados, sugar beets, and other gums. However, due to its structural differences from glucose, it has a higher melting point.
Galactose Formula
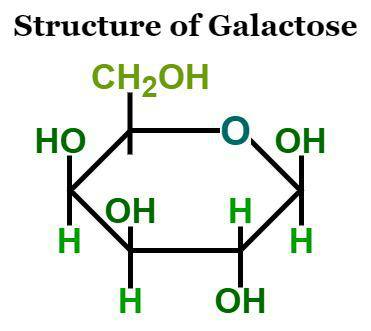
Galactose is a type of sugar with a chemical formula of C 6 H 12 O 6 . It's a six-carbon sugar that looks like a white, odorless powder and tastes sweet. Galactose dissolves easily in water but only a little bit in ethanol. When it combines with another sugar called glucose, it forms a disaccharide called lactose. Galactose is quite similar to glucose in structure, except for one tiny difference in where a certain part is located. It's not as sweet as regular sugar, sucrose, being about 65% as sweet. Galactose is often abbreviated as 'Gal' and is also known as brain sugar.
Galactose is one of the three important sugars in the bodies of mammals, alongside glucose and fructose. You can mostly find it in dairy products, like milk and cheese. But it's also present in avocados, sugar beets, and certain plant substances like gums and mucilage. Galactose is a simple sugar that gives us energy and plays a vital role in creating many big molecules in our bodies. Our bodies can even make galactose from glucose. It can attach itself to fats to make glycolipids and to proteins to make glycoproteins. Plus, it's beneficial for our immune system and helps with digestion.
Galactose Formula Structure
Galactose is a monosaccharide and glucose epimer with hydrogen bond donor and acceptor properties of 5 and 6, respectively. The Molecular Formula is C 6 H 12 O 6
The Galactose Formula is composed of two different sugars: galactose and glucose. It's a type of disaccharide. Louis Pasteur isolated and studied galactose in 1856, and Berthelot renamed it "galactose" or "glucose lactique" in 1860. In 1894, Emil Fischer and Robert Morrell discovered the structure of galactose. It comes in both open-chain and cyclic forms. The open-chain form is characterized by a carbonyl at the end.
Properties of Galactose
Lactose is a simple sugar found in lactose in its D-form and belongs to the simple carbohydrate class. It is a white solid with no odor. Glucose (monosaccharide) is combined with lactose in a condensation reaction to form lactose.
| Properties of Galactose | |
| IUPAC name of Galactose Formula | (3R,4S,5R,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol |
| Molecular formula | C 6 H 12 O 6 |
| Molecular mass | 180 g/mol |
| Density | 1.5 g.cm3 |
| Melting point | -168-170° C |
Also Read: Copper Formula
Galactose Formula Molecular e-structural
There are four isomers with cyclic structures, two containing pyranose (six-membered) rings and two containing furanose (five-membered) rings. Galactofuranose can be found in bacteria, fungi, and protozoa. Its cyclic form has two anomers: alpha and beta. The alcohol group is situated in the equatorial position in the beta form and in the axial position in the alpha form.
Galactose Formula Fructose
Monosaccharides, such as glucose, galactose, and fructose, are isomers (hexoses) with the same chemical formula but varying structures. Glucose and galactose are classified as aldoses, while fructose is a ketose. In aqueous solutions, monosaccharides typically exist in a ring shape. When glucose adopts a ring form, the hydroxyl group around the anomeric carbon (carbon 1) can be arranged in two ways - below the carbon (α position) or above it (β position).
Also Read: Carbon Chemical Formula
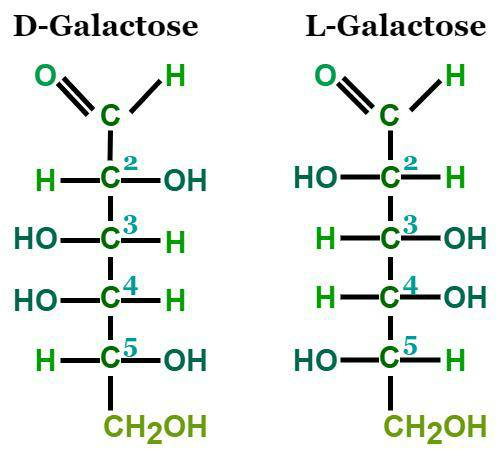
D Galactose Formula
The two enantiomers of galactose are Dextrogalactose (D-galactose) and Levogalactose (L-galactose), which are mirror images of each other. The below figure represents open-chain forms of the enantiomers of galactose, where we can notice a carbonyl at the end of the chain.
Galactose Empirical Formula
The Galactose Empirical Formula is C H 2 O . This formula informs us about the relative ratios of different atoms in a compound or molecule. These ratios of atoms are also valid on the molar level. Because we do not know the number of atoms present in a molecule or compound, we cannot use this formula to make the actual structure of a molecule or compound.
Metabolism of Galactose
In the liver, gal can be converted to glucose l-phosphate and later on to glucose 6-phosphate. There is also a small alternate pathway that has yet to be identified. The first and most crucial step in this process is galactokinase phosphorylation. This enzyme exists in two different forms depending on the genetic makeup, each with its own distribution among different tissues. When galactokinase 1 is defective, cataracts may develop during childhood or early adulthood due to the accumulation of galactitol in the lenses.
The next step in Gal metabolism involves converting UDP into UDP-glucose using UDP-glucose-hexose-i-phosphate uridylyltransferase. This conversion also involves epimerizing UDPGal into UDP-glucose with the help of UDP-glucose-4'-epimerase. This process is similar to an autocatalytic reaction, resulting in a net conversion of Gal 1-phosphate into glucose 1-phosphate as UDP-glucose provides the necessary UDP molecule for the next Gal 1-phosphate molecule. Finally, glucose 1-phosphate is converted into easily metabolized glucose 6-phosphate by a magnesium-dependent enzyme called phosphoglucomutase.
Also Read : Cholesterol Formula
Uses of Galactose
- Galactose, a simple sugar, is essential for the body's metabolism and energy delivery.
- It plays a crucial role during early human development.
- Galactose promotes a healthy digestive system by maintaining beneficial intestinal bacteria.
- This helps improve food digestion and enhances the body's resistance to infections.
- In clinical trials, galactose is used to study treatments and diagnoses for conditions like Hepatitis C, Hepatic Cancer, Wilson's Disease, Diabetic Macular Edema, and Focal Segmental Glomerulosclerosis.
- Galactose serves as a pathway to generate glucose, which is a vital source of energy for the body.
- It is also a component in some commonly used vaccines and non-prescription products.
| Related Links | |
| Sodium Acetate Formula | Chlorine Gas Formula |
| Sodium Citrate Formula | Chromic Acid Formula |
| Glycerol Formula | Hydrogen Formula |
Galactose Formula FAQs
What is the biological importance of galactose?
What is the structural form of galactose?
Is galactose necessary for synthesis lactose?
Can galactose be broken down?










