
It is an inorganic compound that is composed of calcium and iodine and has the chemical formula CaI2. It can be made with calcium oxide, calcium carbonate, or calcium hydroxide in the presence of hydrochloric acid. Find out about calcium iodide's chemical structure, properties, and uses in this short article.
What is Calcium lodide?
A compound called calcium lodide can be used for medicine as well as photography. Calcium is an element in alkaline-earth metals. In addition to being the most abundant metallic element in the human body, calcium is also the fifth most abundant element in the earth's crust. Nevertheless, calcium does not occur naturally in the Free State, even though calcium oxide, CaO, is widespread.Also Check - Value of Gas Constant Formula
Humans have 2% calcium in their bodies and its main sources are milk, milk products, fish, and green leafy vegetables. Calcium Iodide is an inorganic compound. As with similar salts such as calcium chloride, it is colorless, deliquescent, and highly soluble in water. As a source of iodine in cat food, it is also useful in photography.Also Read : Acetone Formula
What is Calcium lodide Formula?
At 200-400 degrees Celsius, calcium reacts with iodine gas to produce calcium iodide compounds. The ionic compound Calcium Iodide is formed when calcium and iodine bond together. This happens because calcium loses two valence electrons, becoming Ca2+ and creating a positive charge. In order to form the bond, iodine gains one electron, becoming I−. The formula for calcium iodide is CaI2, indicating that two atoms of iodine are needed to balance out the loss of electrons from one atom of calcium. Additionally, the formation of calcium iodide involves a redox reaction where calcium is oxidised to Ca2+ and iodine (I) is reduced to iodide (I−).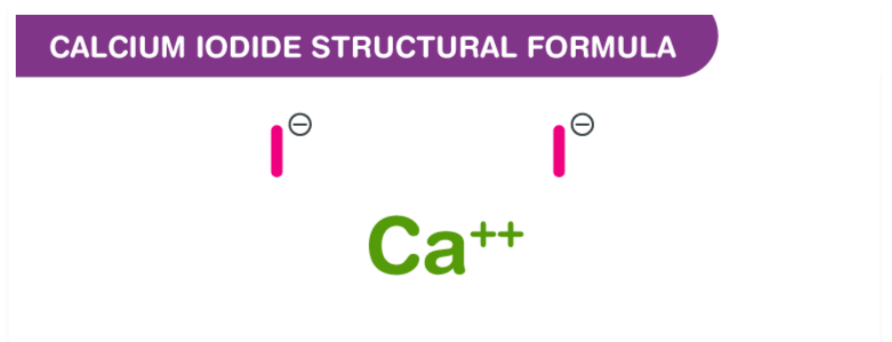
Properties Of Calcium lodide Formula
It has a white color. Its melting point is 779 degrees C. It is easily soluble in acetone and alcohol. It boils at 1100 degrees C. When it is acidically attacked, hydrogen iodide is released. Dehydrated chemical compounds for it are CaI2. 2H2O with a molecular weight of 330.02. It is mostly used in photography, pharmaceuticals, and analysis reagents.| Properties of Calcium Iodide | |
| Name | Calcium Iodide |
| Also Known as | Calcium (II) Iodide |
| Appearance | White Solid |
| Molecular Formula | CaI2 |
| Melting Point | 779 °C |
| Boiling Point | 1100 °C |
| Density | 3.956 g/cm3 |
| Molar Mass | 293.887 g/mol |
| Solubility in Water | Soluble |
Chemical Properties of Calcium lodide
- In addition to water, calcium iodide is highly soluble in ethanol, methanol, and acetone, which are polar solvents.
- Since calcium iodide is a hygroscopic compound, it readily absorbs humidity from the environment, forming hydrates such as dihydrates.
- By donating electrons, calcium iodide reduces metal oxides to their corresponding metals. For example, copper oxide is reduced to copper by reacting with calcium iodide.
- Hydrochloric acid (HCl) reacts with calcium iodide to form calcium chloride (CaCl2).
- Hydrogen iodide (HI) and sodium iodide (NI).
- In halogen displacement reactions, calcium iodide displaces halogen from their salts. For example, with silver nitrate (AgNO3).
- Silver ions can be displaced by calcium iodide to form silver iodide (AgI) and calcium nitrate (Ca(NO3)2).
Also Check - Ascorbic Acid Formula
Calcium lodide Uses
The following are some of the main uses of this chemical compound.- In food additives, pharmaceuticals, and photographic chemicals, calcium iodide is used as a source of iodine.
- Various organic chemical reactions, such as Birch reduction, use calcium iodide as a good reducing agent.
- By reducing the thyroid's supply of iodine, it is used to treat hyperthyroidism caused by an overactive thyroid gland.
- In addition to humans, it is added to animal feed as a source of iodine since it is essential to the thyroid gland's proper function.
- In some nuclear reactors, calcium iodide is used as a scintillator, a material that produces light when radiation strikes. This light can then be detected and used to measure radiation levels.
Calcium Iodide Formula FAQs
What is the chemical formula for Calcium Iodide?
The chemical formula for Calcium Iodide is CaI2, indicating that it consists of one calcium (Ca) atom bonded to two iodine (I) atoms.
Is Calcium Iodide soluble in water?
Yes, Calcium Iodide is soluble in water, and it dissolves readily to form a clear solution.
What are the common uses of Calcium Iodide?
Calcium Iodide is used in various applications, including as a nutritional supplement to provide iodine, in the preparation of photographic emulsions, and in some organic synthesis reactions.
Is Calcium Iodide considered safe for human consumption?
Calcium Iodide is generally safe when used as a dietary supplement to provide essential iodine. However, it should be used under recommended guidelines and doses to prevent excessive iodine intake.
What are the potential health effects of Calcium Iodide exposure?
In normal use and handling, Calcium Iodide exposure is not typically associated with significant health risks. However, like many chemicals, it should be handled with care to avoid skin and eye irritation, and excessive consumption should be avoided due to potential iodine toxicity.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









