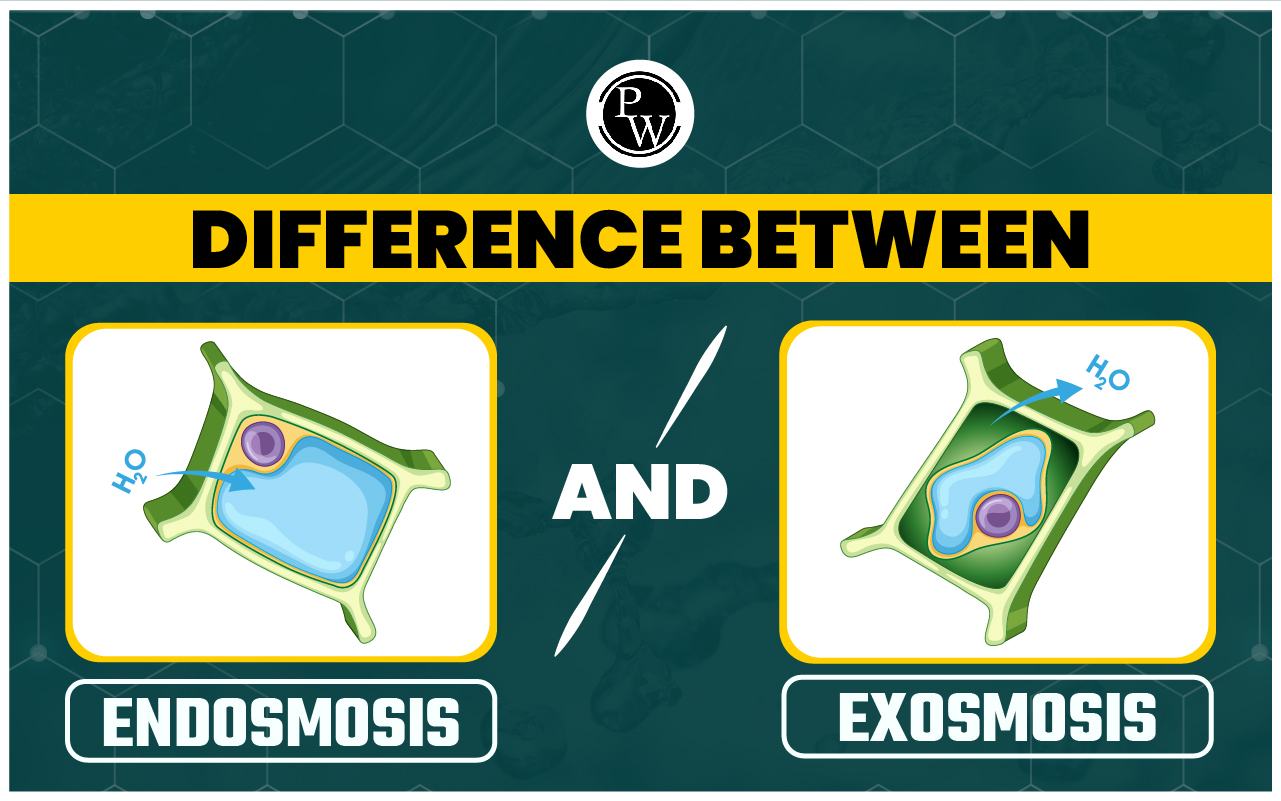
Difference Between Endosmosis And Exosmosis: Endosmosis and exosmosis are two different types of osmosis. The fundamental distinction is in their directionality: endosmosis involves the inward movement of water within the cell, whereas exosmosis involves the outward expulsion of water from it. The terms hypertonic, hypotonic, and isotonic solutions aid in the understanding of these processes. In a hypertonic solution, the solvent has a higher solute concentration than the cell, making the cell hypotonic to the solvent, whereas in a hypotonic solution, the cell sap has a lower solute concentration than the solvent, making the cell hypertonic to the solution. The solution and the cell sap are isotonic when the solute concentrations are equal.
| NEET Biology Syllabus | NEET Biology Diagrams |
| NEET Biology MCQ | NEET Biology Chapter wise Weightage |
| NEET Biology Notes | NEET Previous Year Question papers |
Endosmosis occurs when cells are immersed in hypotonic solutions, which causes cell expansion. Exosmosis occurs when cells are exposed to hypertonic solutions, causing them to shrink. Isotonic solutions do not promote exosmosis or endosmosis. Refer to the article below for mor e details on the difference between endosmosis and exosmosis.
Difference Between Endosmosis And Exosmosis Overview
Osmosis occurs when water flows across a semipermeable membrane from an area with fewer dissolved particles to an area with more dissolved particles until they balance. There are two kinds of osmosis: endosmosis and exosmosis. Endosmosis occurs when water moves from the outside of a cell to its interior or cytoplasm. Place a cell in a hypotonic solution to observe endosmosis. More dissolved substances are inside the cell than outside in a hypotonic solution. However, because only water can pass through the membrane, it moves from its higher concentration outside the cell to the cytoplasm. This causes the cell to swell, become turgid, and even burst. Exosmosis occurs when water flows away from a cell or vessel. This happens when there is a difference in water potential between inside and outside the cell. In this case, dissolved particles outside the cell are higher than inside. Exosmosis occurs when water leaves the cell through the membrane, causing the cell to shrink and undergo plasmolysis. The article below details about the difference between endosmosis and exosmosis in tabular form.Difference Between Endosmosis And Exosmosis
Endosmosis and exosmosis are two types of osmosis in which water moves in and out of cells. Endosmosis is when water moves into a cell, while exosmosis is when water moves out of a cell. The table shows the main difference between endosmosis and exosmosis:| Difference Between Endosmosis And Exosmosis | ||
|---|---|---|
| Aspect | Endosmosis | Exosmosis |
| Definition | Movement of molecules from high to low concentration | Movement of molecules from low to high concentration |
| Examples | Nutrient and water intake by plant cells | Release of waste products from plant cells |
| Mechanism | Driven by concentration gradient across membrane | Driven by pressure differential across membrane |
| Significance | Allows cells to absorb nutrients and water | Facilitates elimination of waste products from cells |
| Consequences | Risk of cell enlargement or bursting in excess | Beneficial for toxin removal in excess |
| Effect on Water | Decreases water potential | Increases water potential |
| Solution | Hypotonic solution | Hypertonic solution |
| Solvent Movement | Enters the cell | Exits the cell |
| Required Solution | Hypotonic | Hypertonic |
| Osmotic Pressure | Pulls water into the cell | Pushes water out of the cell |
Endosmosis
Endosmosis refers to the process where water moves through a semipermeable membrane into an area with a higher concentration of solutes. Simply put, it occurs when water flows from a region with less solute concentration to one with more solutes. This occurs when two solutions are separated by a semipermeable membrane, with the solution having the higher solute concentration surrounding the one with the lower concentration. Endosmosis is a specific type of osmosis, which is water movement across a semipermeable membrane. Examples include water movement across cell membranes, bacterial membranes, and plant cell walls. Osmosis relies on specific conditions, including the concentration of solutes and solvents both inside and outside the cell during endosmosis:- The solvent concentration is higher outside the cell, while the solute concentration is lower inside the cell.
- The cytoplasm within the cell has a higher concentration of solutes and a lower solvent concentration than its surroundings.
Exosmosis
Exosmosis refers to the process of water moving from an area where it's highly concentrated to an area where it's less concentrated. This movement occurs across a semipermeable membrane, which only allows water to pass through, not solutes. As water moves, it exerts pressure on the side with higher concentration, known as osmotic pressure. Examples include water leaving a cell when the surrounding solution has fewer solutes and water exiting bacteria under similar conditions. Exosmosis relies on the following conditions- In exosmosis, the concentration of solutes is higher outside the cell than inside.
- Meanwhile, the solvent concentration (water) is lower outside and higher inside the cell.
What is difference between endosmosis and exosmosis?
How does endocytosis differ from endosmosis?
Can you give an example of endosmosis?
What's the process of endosmosis and exosmosis?
Is potato osmosis an example of endosmosis?