
Ionic Equilibrium Notes for NEET: Ionic equilibrium holds significant importance in the NEET 2025 exam as it is a high-weightage topic in the chemistry section. Many questions related to pH calculations, buffer systems, and solubility equilibrium are directly asked, often involving problem-solving skills. A strong grasp of this chapter enables NEET aspirants to confidently tackle numerical problems and theoretical questions, improving their overall score.
Additionally, ionic equilibrium links closely with other crucial chapters like thermodynamics, electrochemistry, and chemical equilibrium, making it a cornerstone of physical chemistry. Mastery of this topic not only aids in scoring well but also enhances understanding of advanced concepts needed for medical studies.
Also Check:
Ionic Equilibrium Notes for NEET Overview
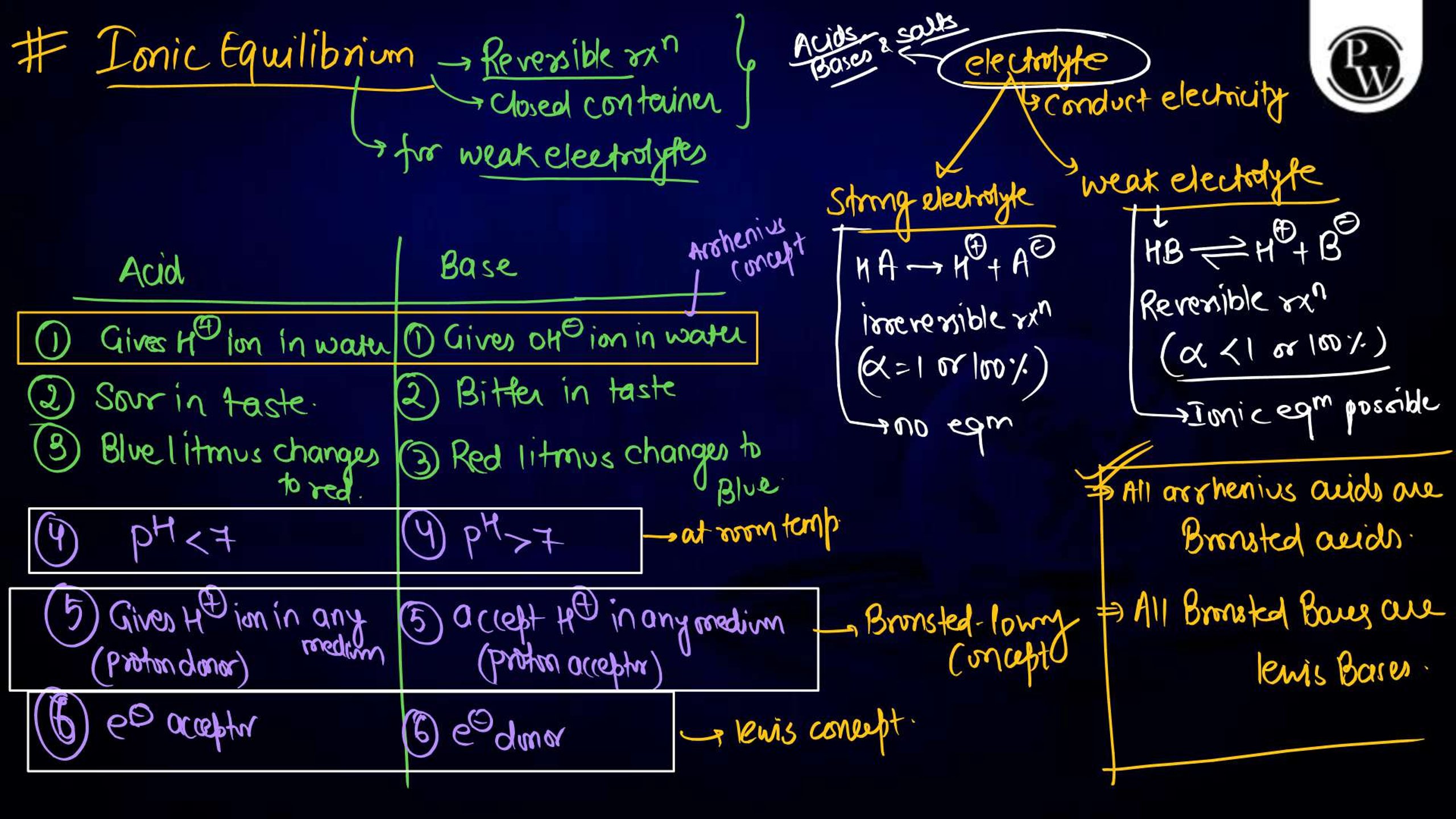
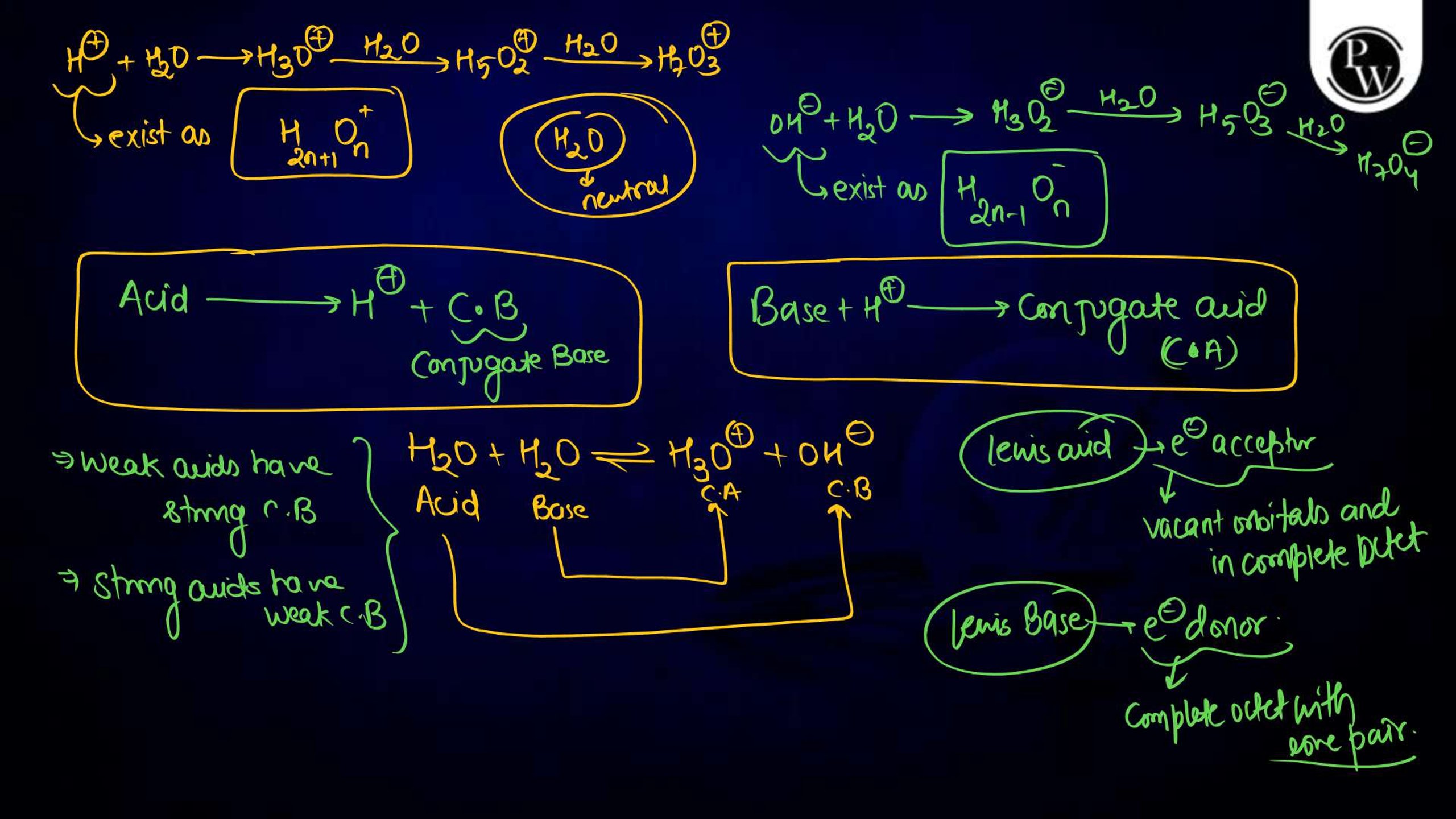
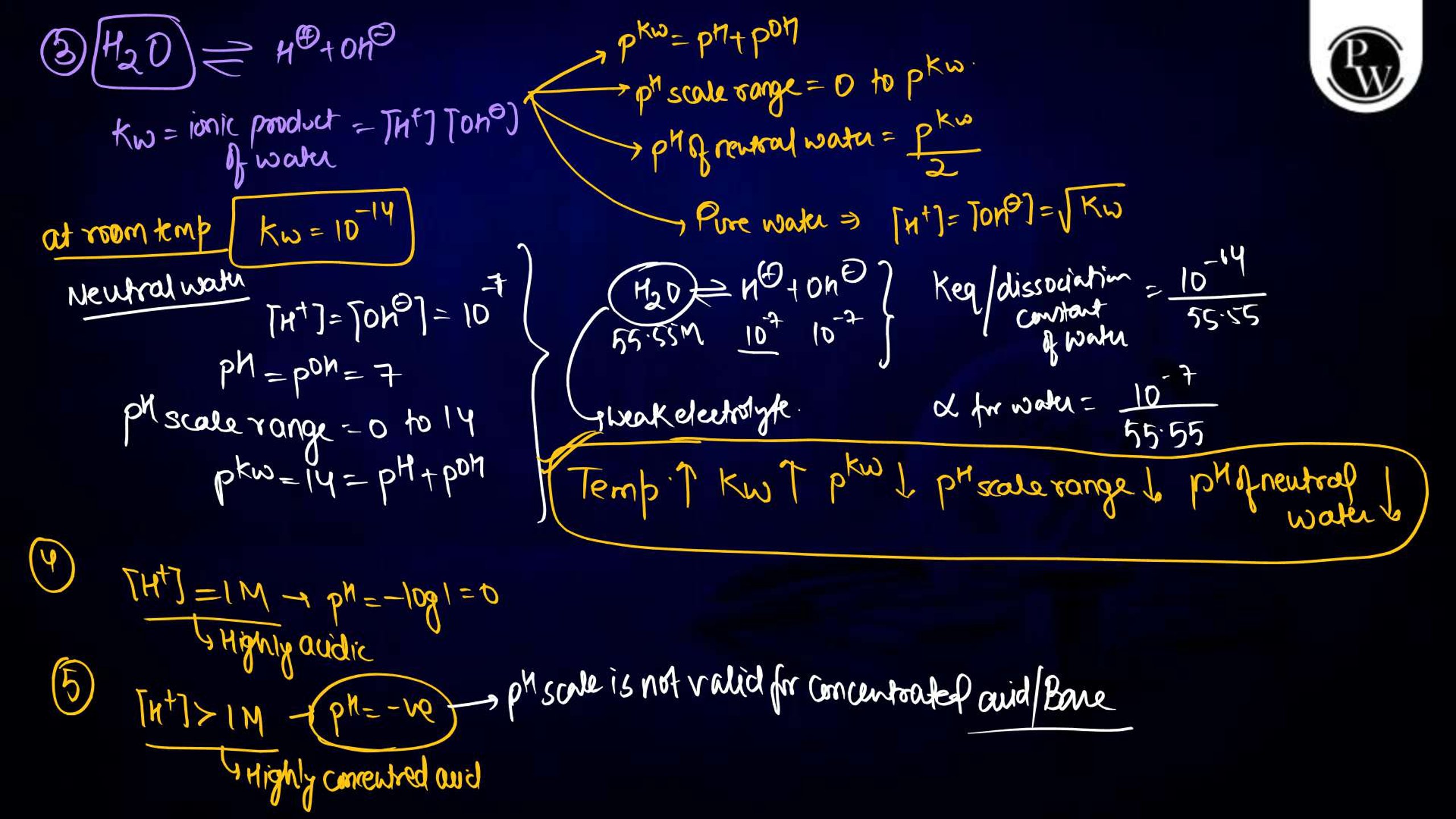
Ionic equilibrium is a vital concept in physical chemistry , particularly for NEET aspirants. It deals with the equilibrium established between ions in a solution during a chemical reaction. Understanding ionic equilibrium is essential for solving problems related to pH, buffer solutions, solubility products, and acid-base equilibria. Class notes on ionic equilibrium provide a concise, well-structured guide to help students comprehend key principles, formulas, and applications. These notes simplify complex topics, aiding NEET aspirants in mastering this significant portion of the syllabus effectively.



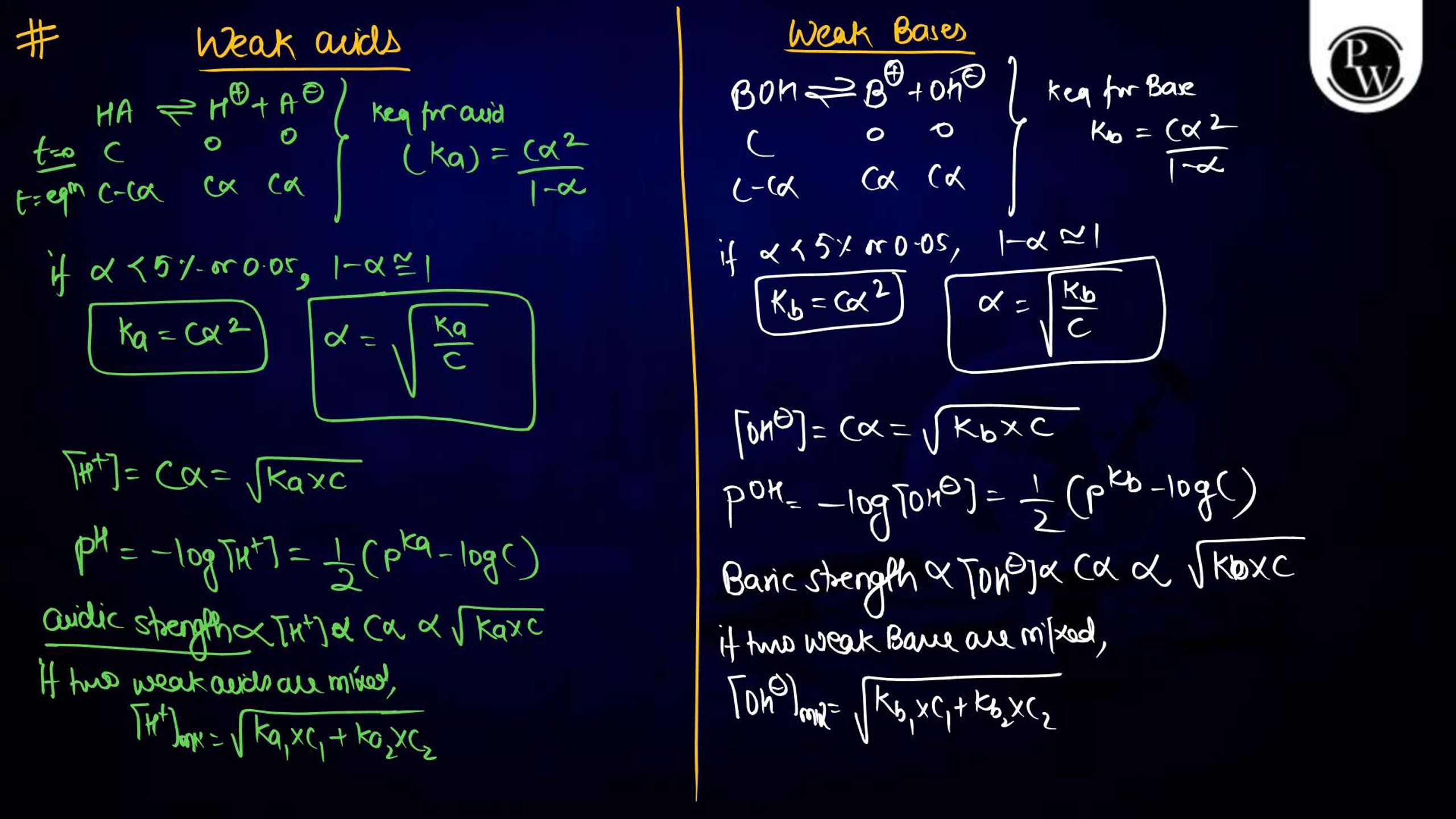
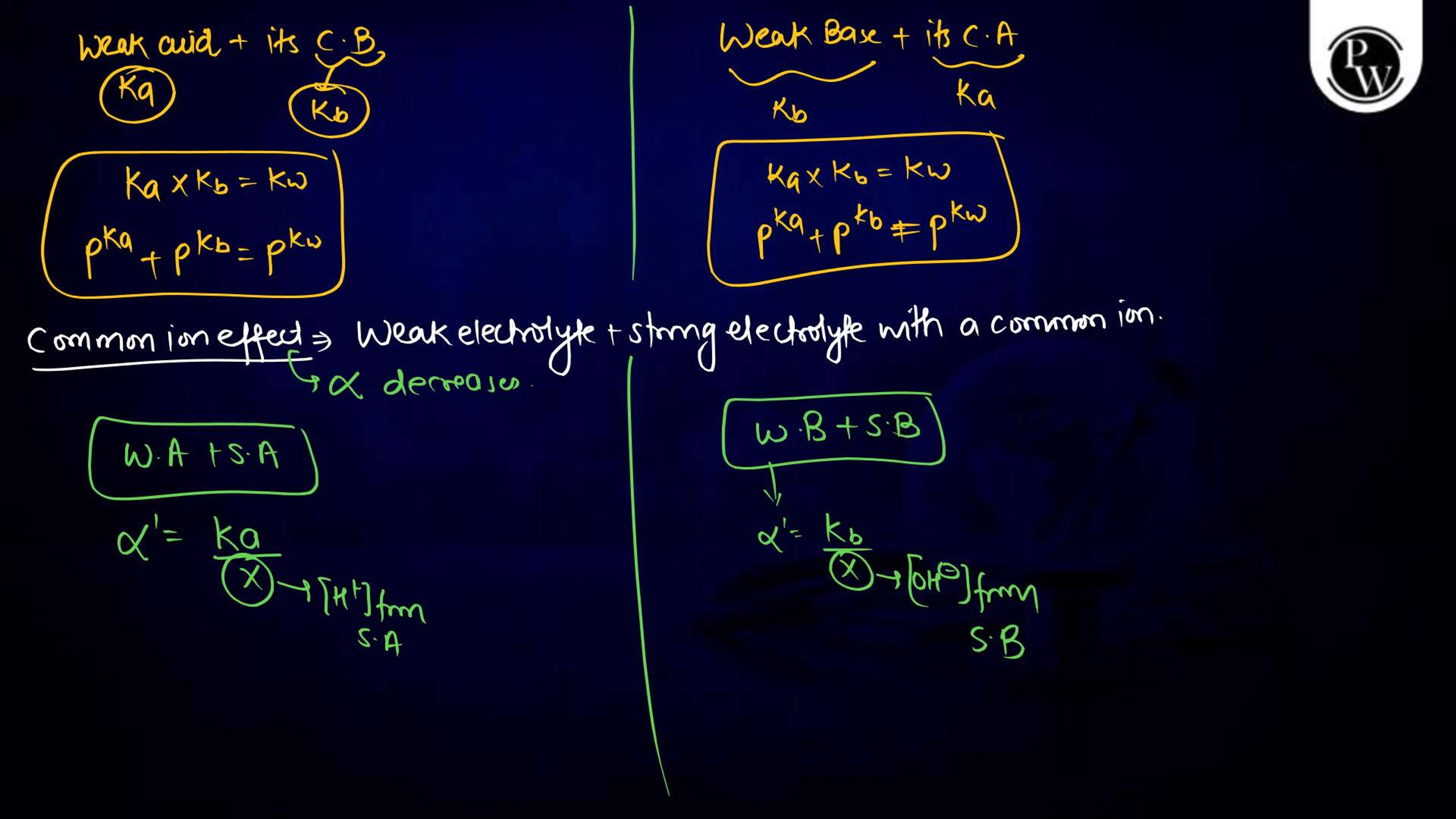
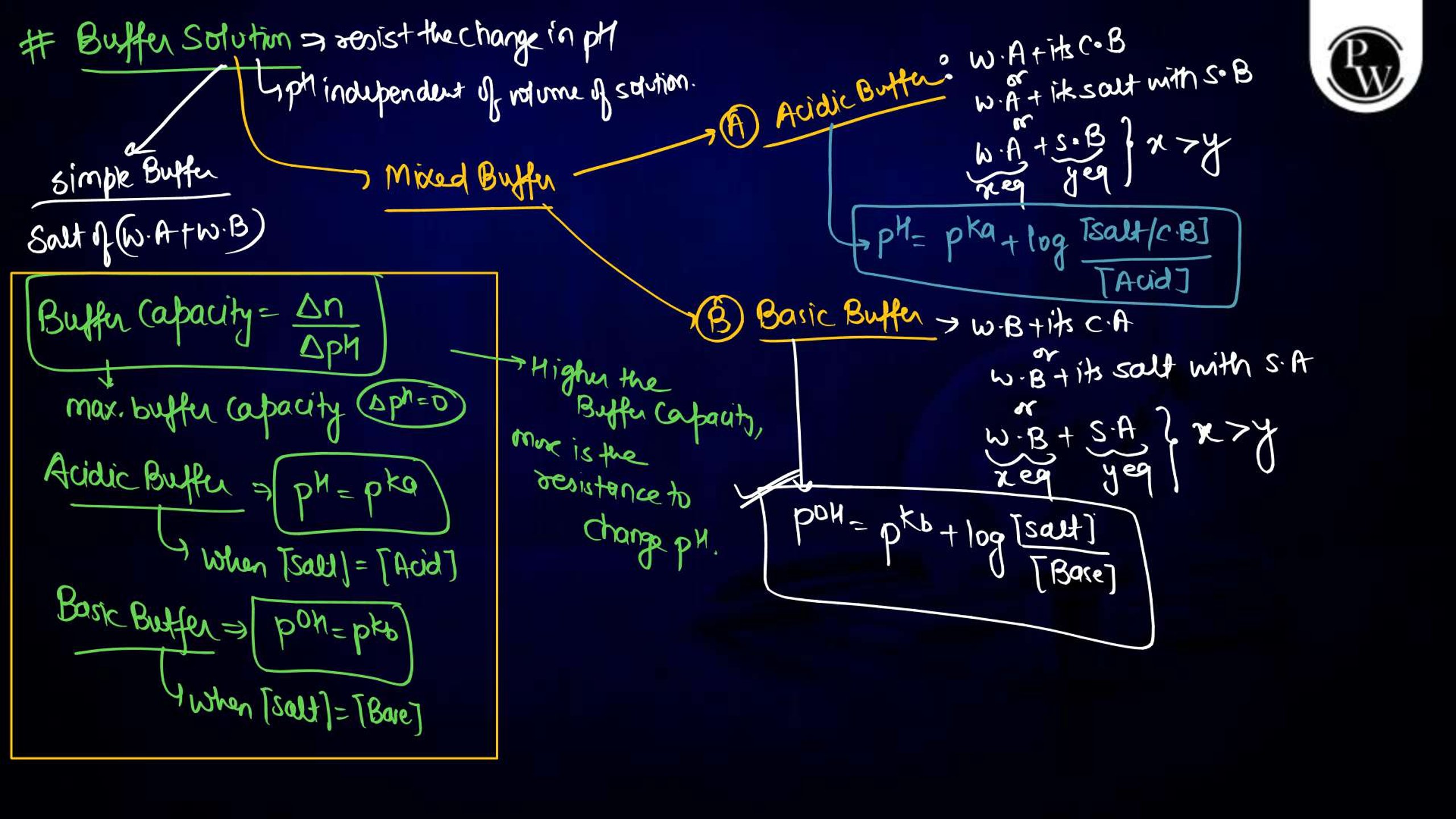
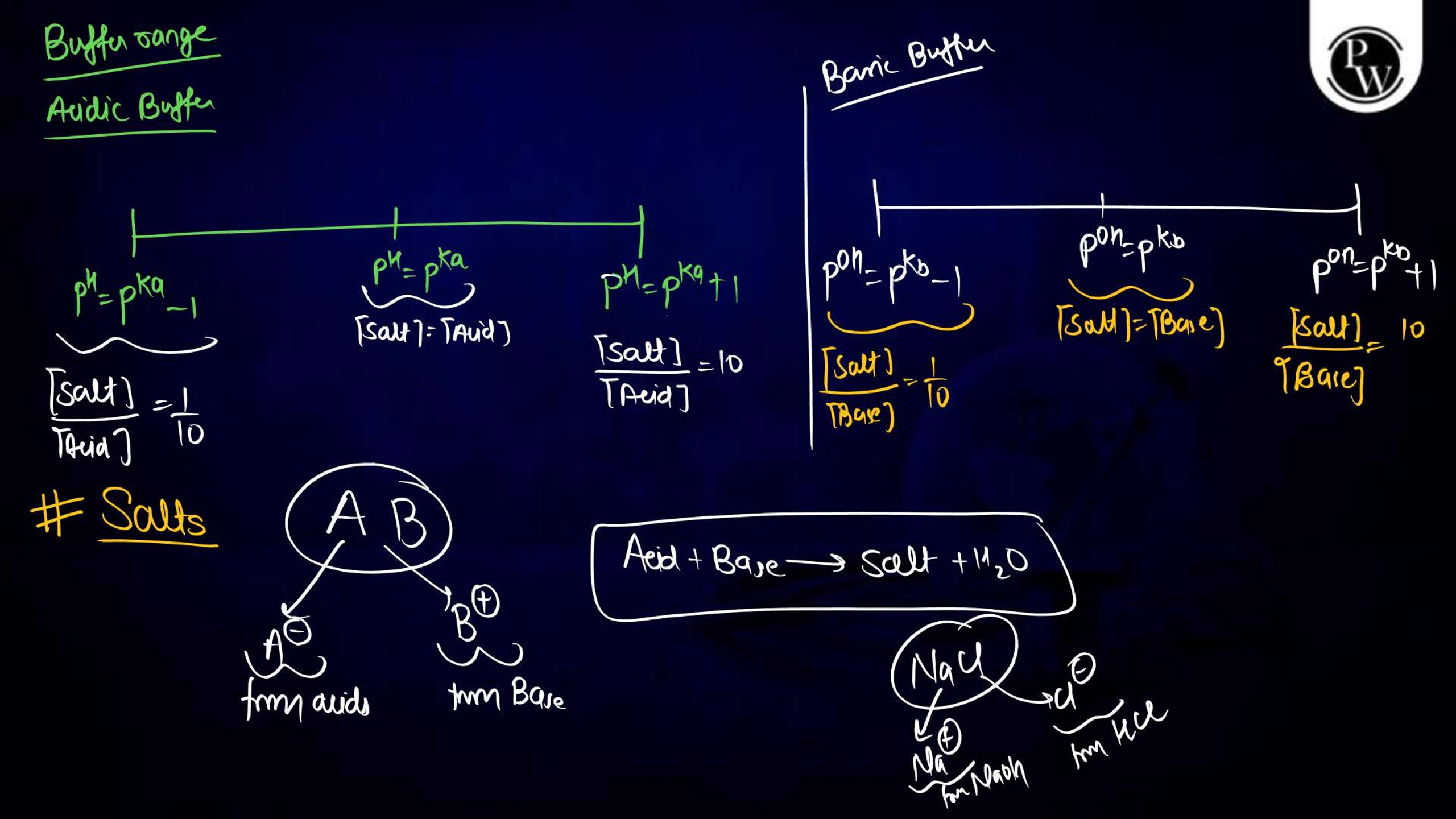
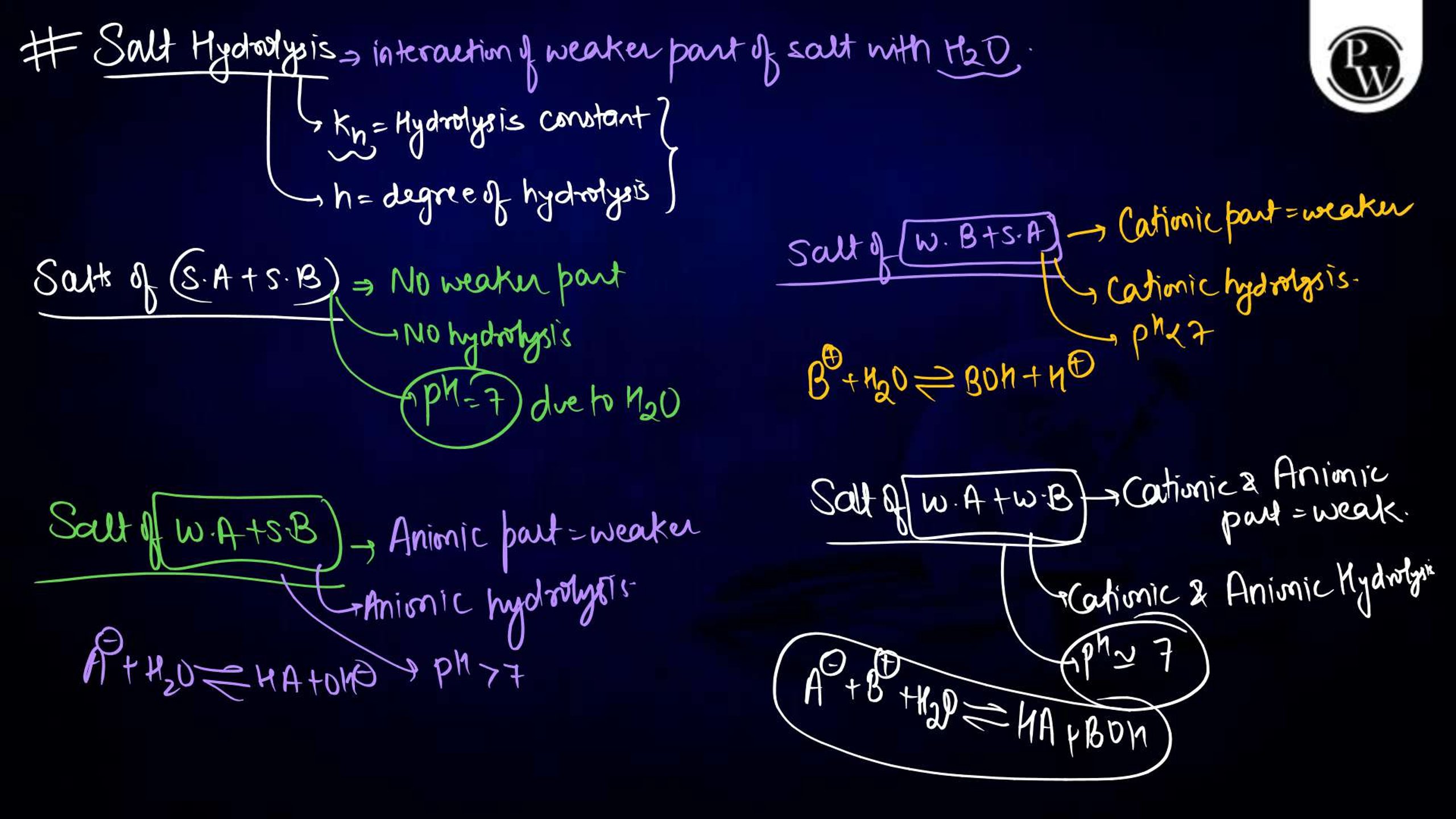

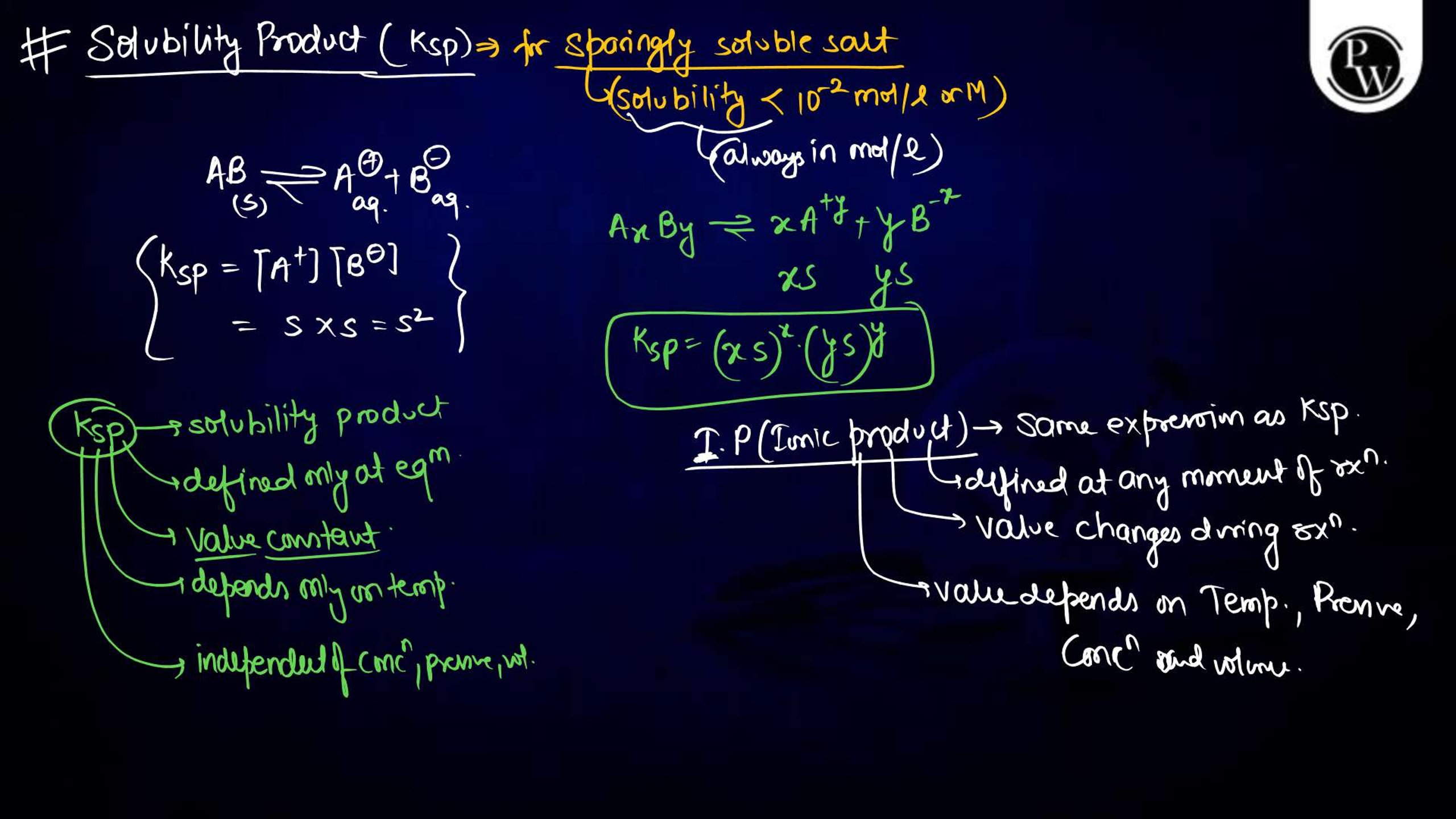

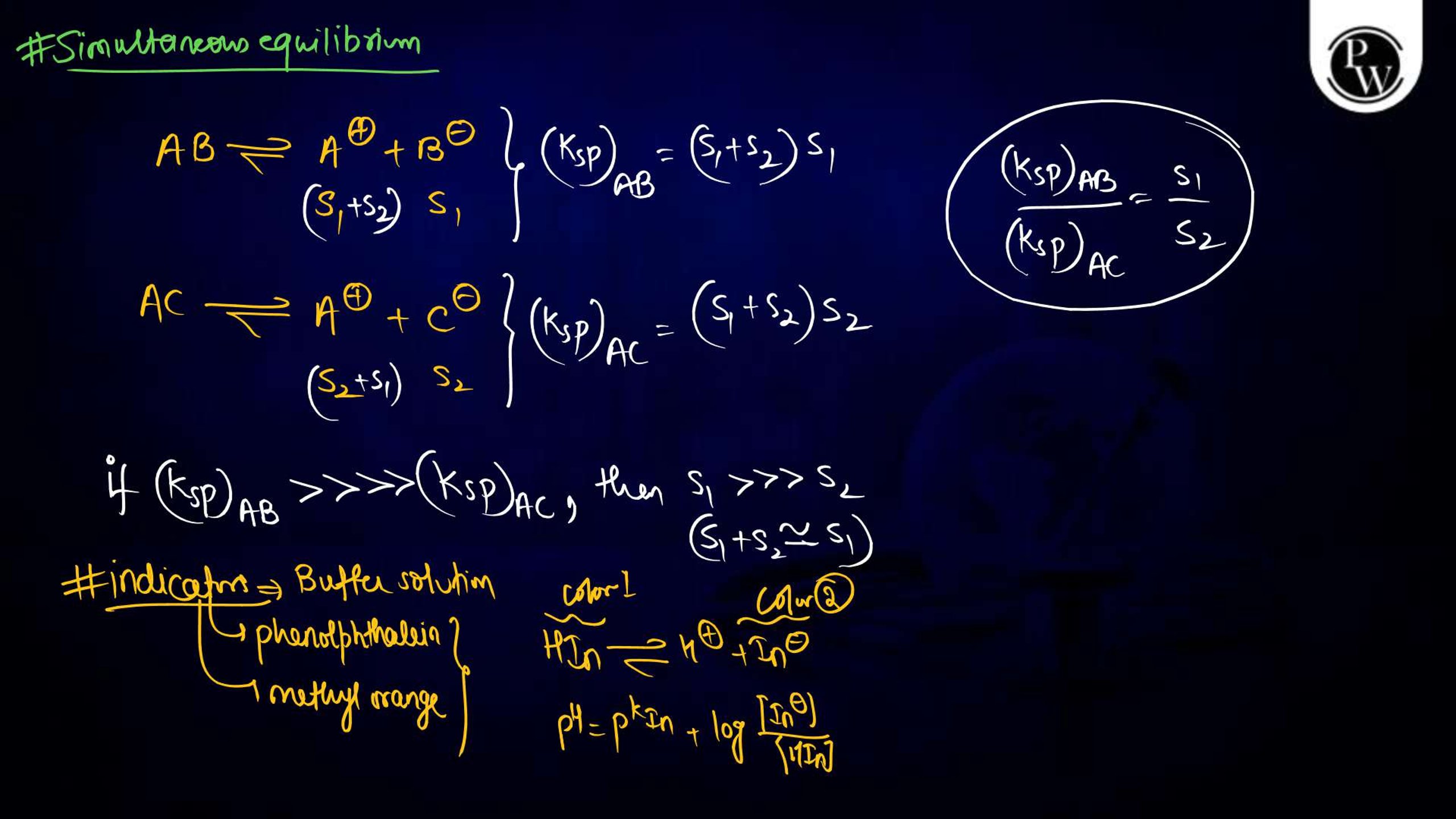
Ionic Equilibrium Notes PDF for NEET
The Ionic equilibrium notes are available in a downloadable PDF format, covering all the key points mentioned above. The Ionic Equilibrium Notes for NEET concise yet detailed, including diagrams, solved examples, and exam-specific tips. It is an excellent resource for quick revision and problem practice, making it an indispensable part of your NEET preparation.
Download the Ionic Equilibrium Notes for NEET (PDF)
For a more detailed understanding of the entire chapter, a video on Ionic Equilibrium is available on YouTube. The link to the video is provided below.
[embed]https://www.youtube.com/watch?v=kfqIfFxb25g[/embed]Meaning of Ionic Equilibrium
Ionic equilibrium refers to the state of balance achieved in a solution when the rates of dissociation and recombination of ions are equal. It occurs in reversible reactions involving weak acids, weak bases, or sparingly soluble salts. The equilibrium can be described using the law of mass action, which establishes the equilibrium constant, K eq. Ionic equilibrium is crucial in understanding the behavior of substances in solutions and plays a significant role in fields like biochemistry, environmental science, and analytical chemistry.
Weightage of Ionic Equilibrium Notes for NEET - Last 5 Years
Ionic equilibrium holds substantial weightage in the NEET chemistry section. Questions from this topic generally range between 3 to 4 marks, which can be decisive for a student’s rank. Previous year papers reveal that concepts like pH calculations, buffer action, solubility product, and titration curves frequently appear in exams.
|
Weightage Following Previous Year’s Trends |
|||||||
| Chapter Name | 2019 | 2020 | 2021 | 2022 | 2023 | Total 5 yrs PYQ | Average Count |
| Equilibrium | 3 | 2 | 2 | 2 | 2 | 11 | 2 |
The chapter-wise weightage for Chemistry in NEET 2025 is presented below in a tabular format:
| NEET Chapter Wise Weightage 2025 for Chemistry | ||
|---|---|---|
| Topics | Average No. of Questions Based on 5 Years of Analysis | Weightage (%) |
| Equilibrium | 2 | 6% |
Importance of Ionic Equilibrium Notes for NEET
Ionic Equilibrium Notes for NEET are crucial for NEET preparation as they:
- Streamline concepts: The notes break down intricate theories into digestible parts, helping students focus on key ideas.
- Enhance problem-solving skills: With numerous examples, students gain confidence in tackling numerical problems efficiently.
- Support revision: Condensed and organized content ensures a quick and effective review before exams.
- Boost accuracy: These notes emphasize conceptual clarity, reducing the likelihood of errors during the exam.
Tips for Ionic Equilibrium Notes for NEET
To master Ionic Equilibrium, use these strategies to develop a strong understanding and effectively retain essential concepts:- Understand fundamental concepts: Grasp the basics of acids, bases, and salts, along with their dissociation processes.
- Memorize formulas: Key formulas like Ka , Kb, Kw , and Henderson-Hasselbalch equations should be thoroughly learned.
- Practice numerical problems: Regular practice of pH calculations, buffer solutions, and solubility product problems enhances speed and accuracy.
- Focus on common misconceptions: Pay attention to areas like the difference between strong and weak electrolytes, and the effects of dilution on equilibrium.
- Revise regularly: Periodic review of class notes consolidates understanding and ensures better retention of concepts.
Unlock your path to success with PW's comprehensive NEET preparation resources like useful guidance, personalized study plans, mock tests, and PW Online NEET Coaching designed to help you achieve your dream medical college.
| NEET Exam Important Links | |
|---|---|
| NEET Syllabus | NEET Biology Notes |
| NEET Eligibility Criteria | NEET Exam Pattern |
| NEET Previous Year Question Papers | NEET Biology Syllabus |
Ionic Equilibrium Notes for NEET FAQs
Is ionic equilibrium easy for NEET?
What is the note on ionic equilibrium?
Is ionic equilibrium a tough chapter?
What is equilibrium notes Class 11 for NEET?










