
Structure of Atom NEET Notes: The structure of an atom is one of the most fundamental topics in chemistry, especially for students preparing for competitive exams like the NEET 2026 Exam . Understanding the basic building blocks of matter lays the foundation for various concepts in physics, chemistry, and biology. This chapter explains the intricate details of atoms, their components, and how these principles apply to real-world phenomena.
Also Check:
Structure of Atom NEET Notes Overview
The Structure of Atom chapter explores the internal composition of atoms, including protons, neutrons, and electrons. It discusses atomic models proposed by various scientists such as J.J. Thomson, Rutherford, and Bohr. Key concepts include the dual nature of matter, quantum numbers, electron configurations, and the development of modern atomic theory. These topics are essential for solving NEET questions efficiently.



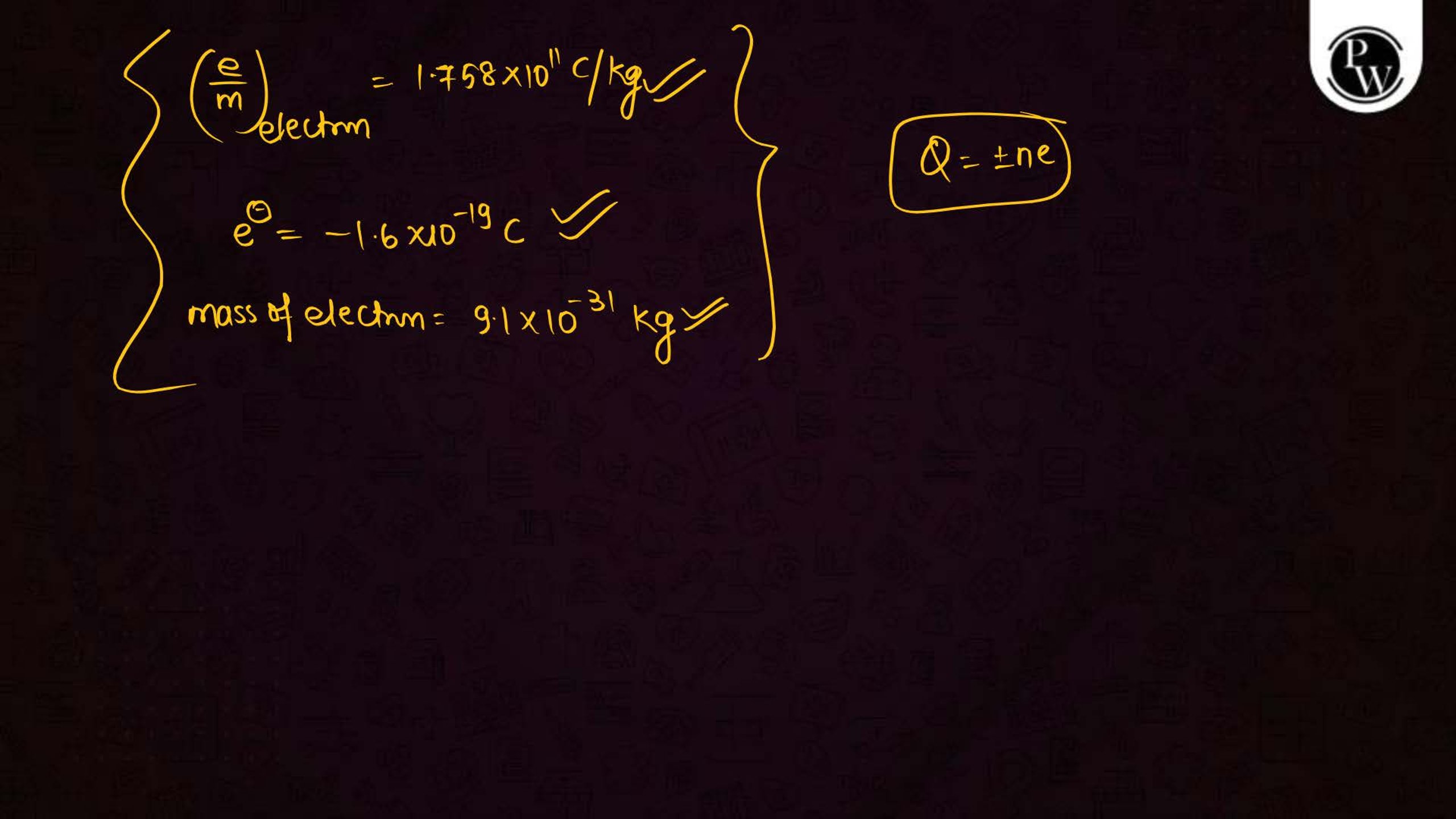




Structure of Atom NEET Notes PDF
For detailed study material, NEET aspirants can access the Structure of Atom NEET Notes PDF provided below. This PDF contains a comprehensive explanation of each topic, making it easier for students to grasp and revise.
Download the PDF of Structure of Atom NEET Notes
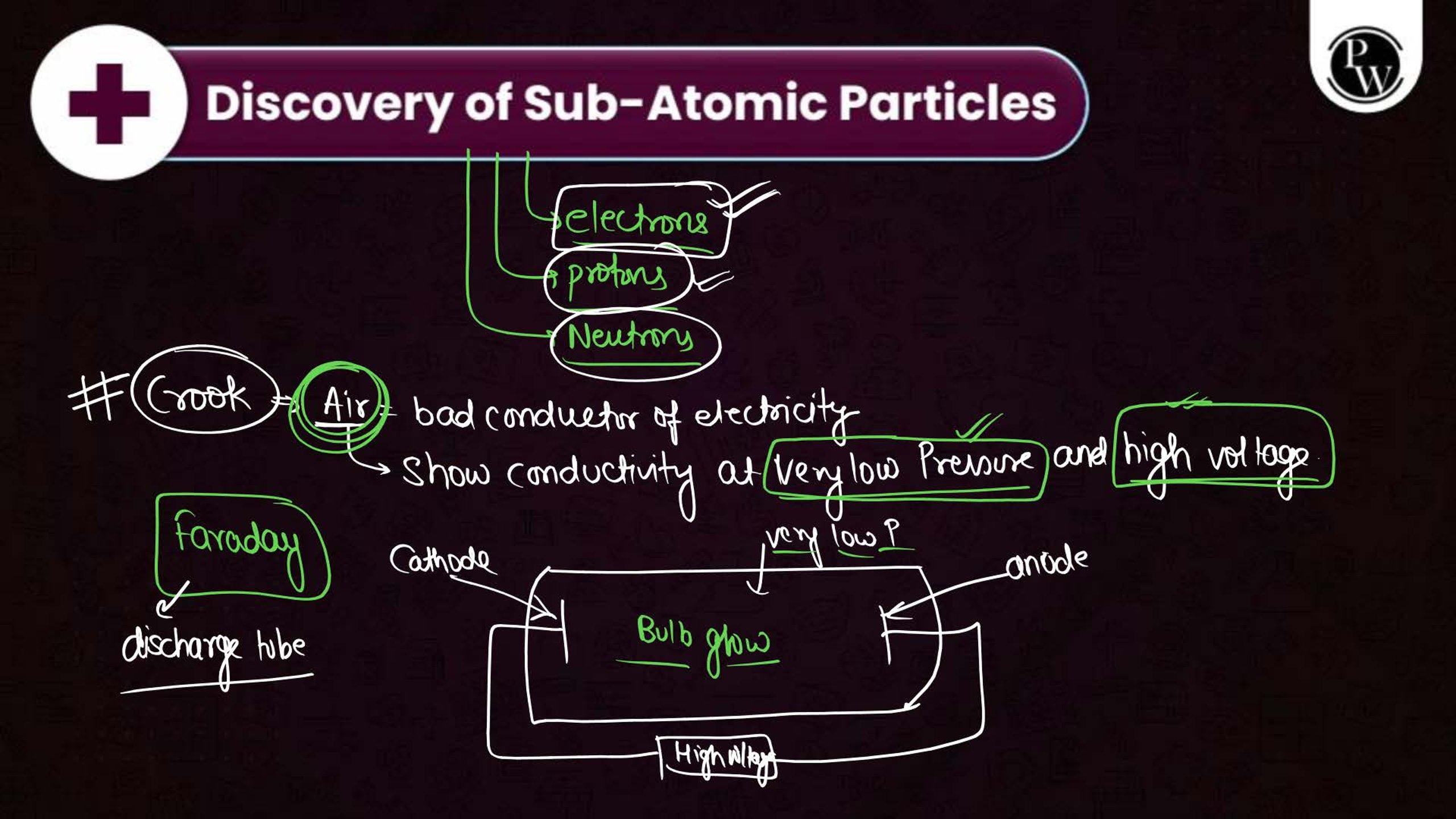
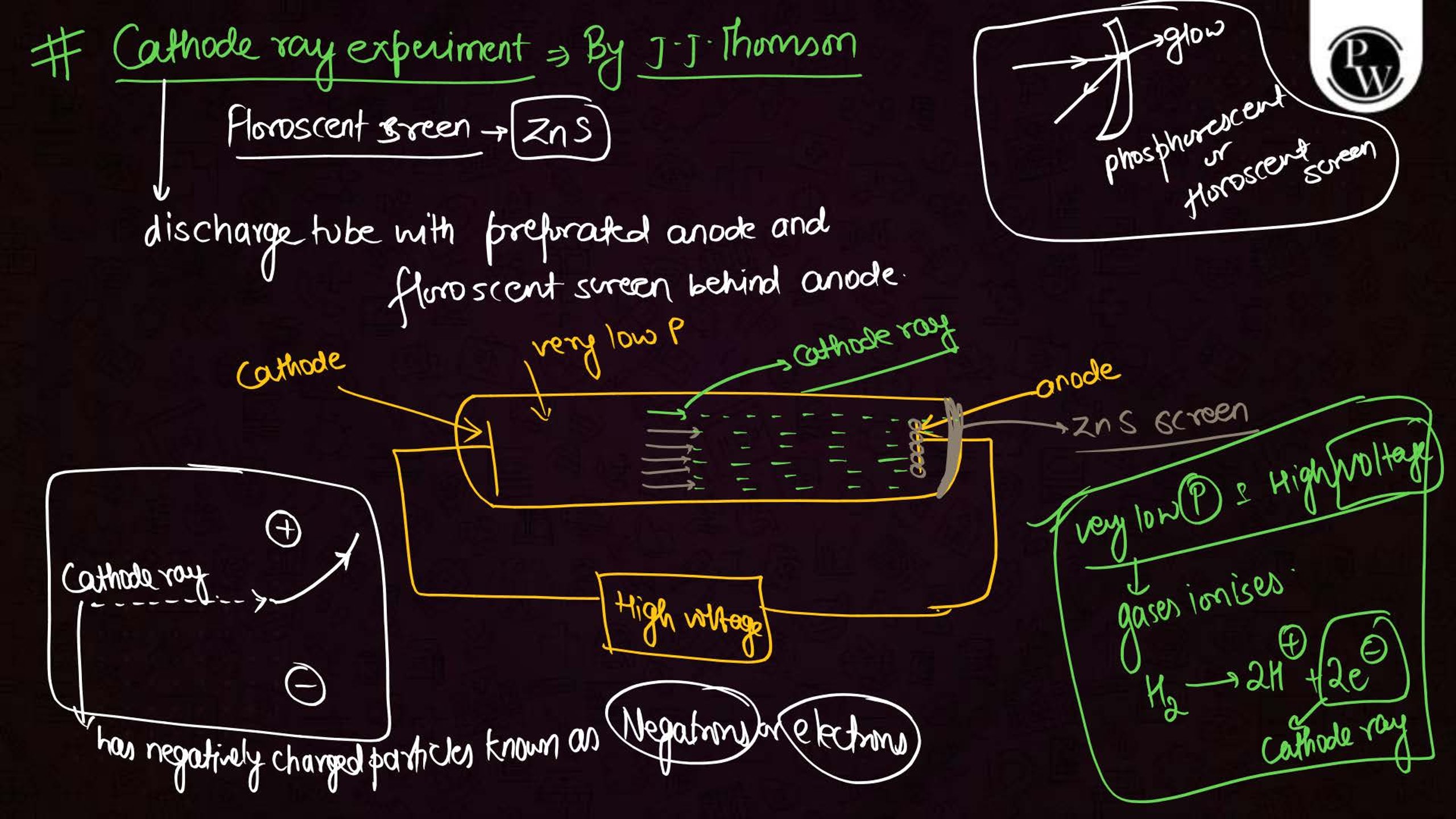
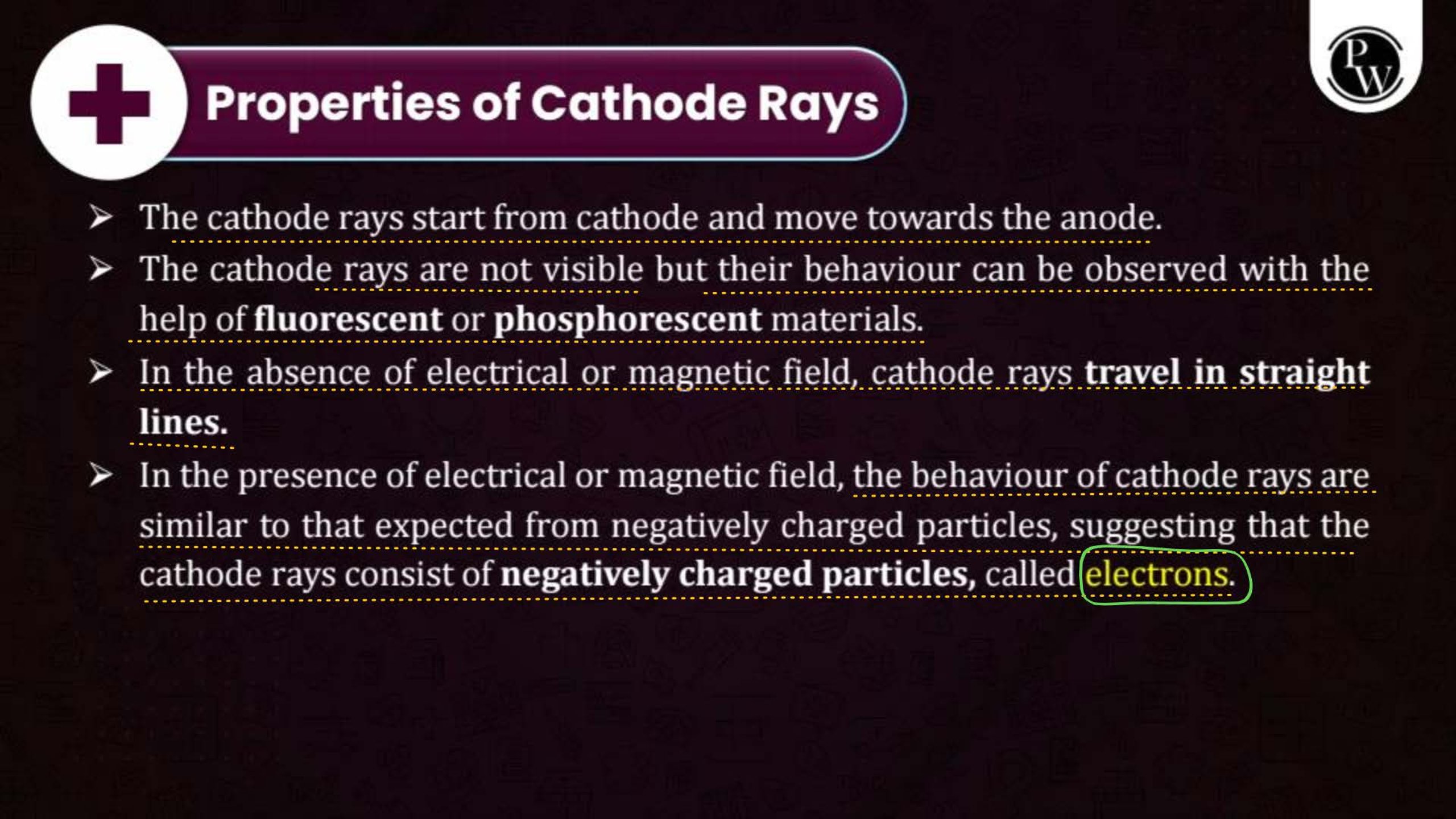
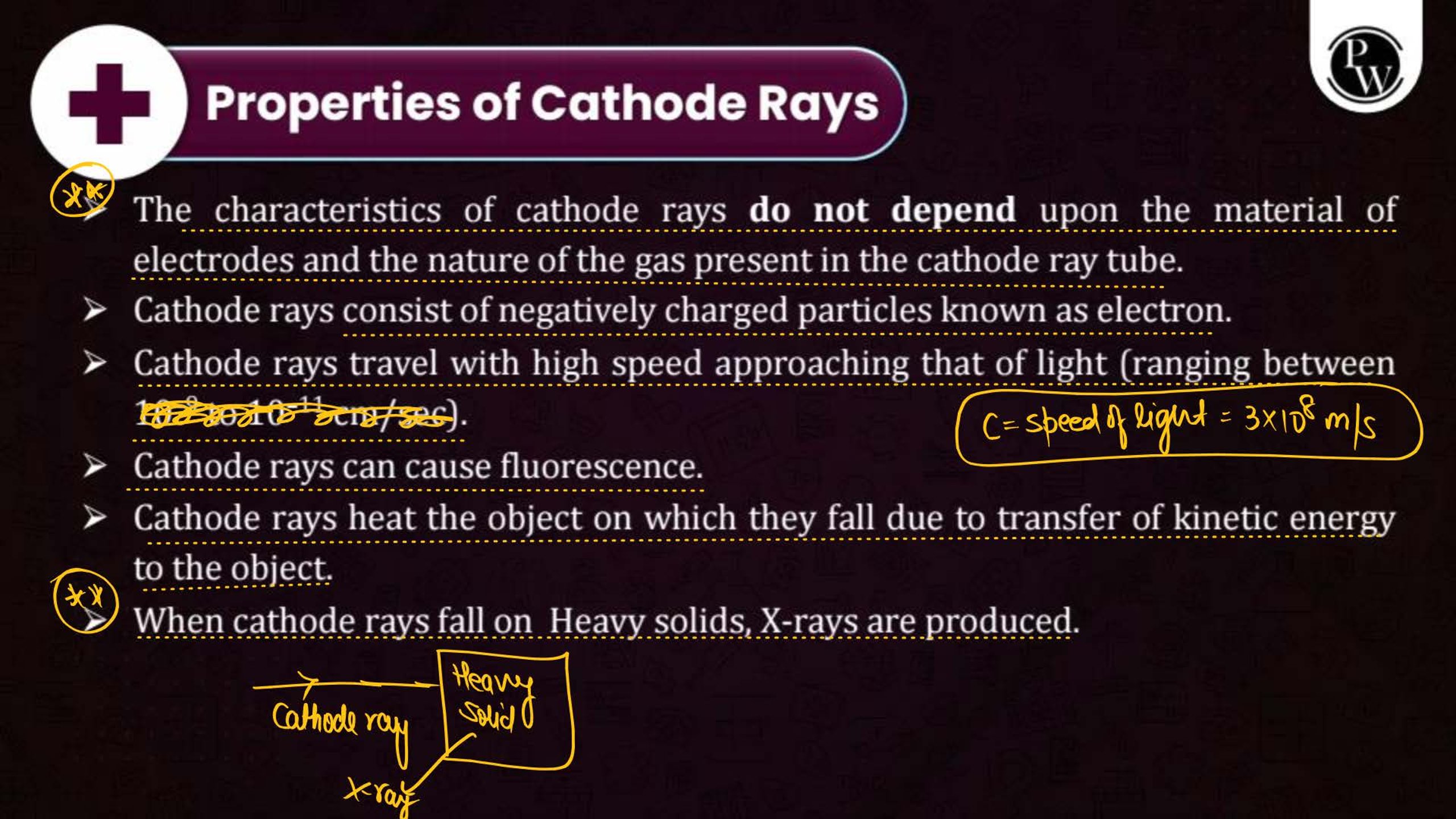
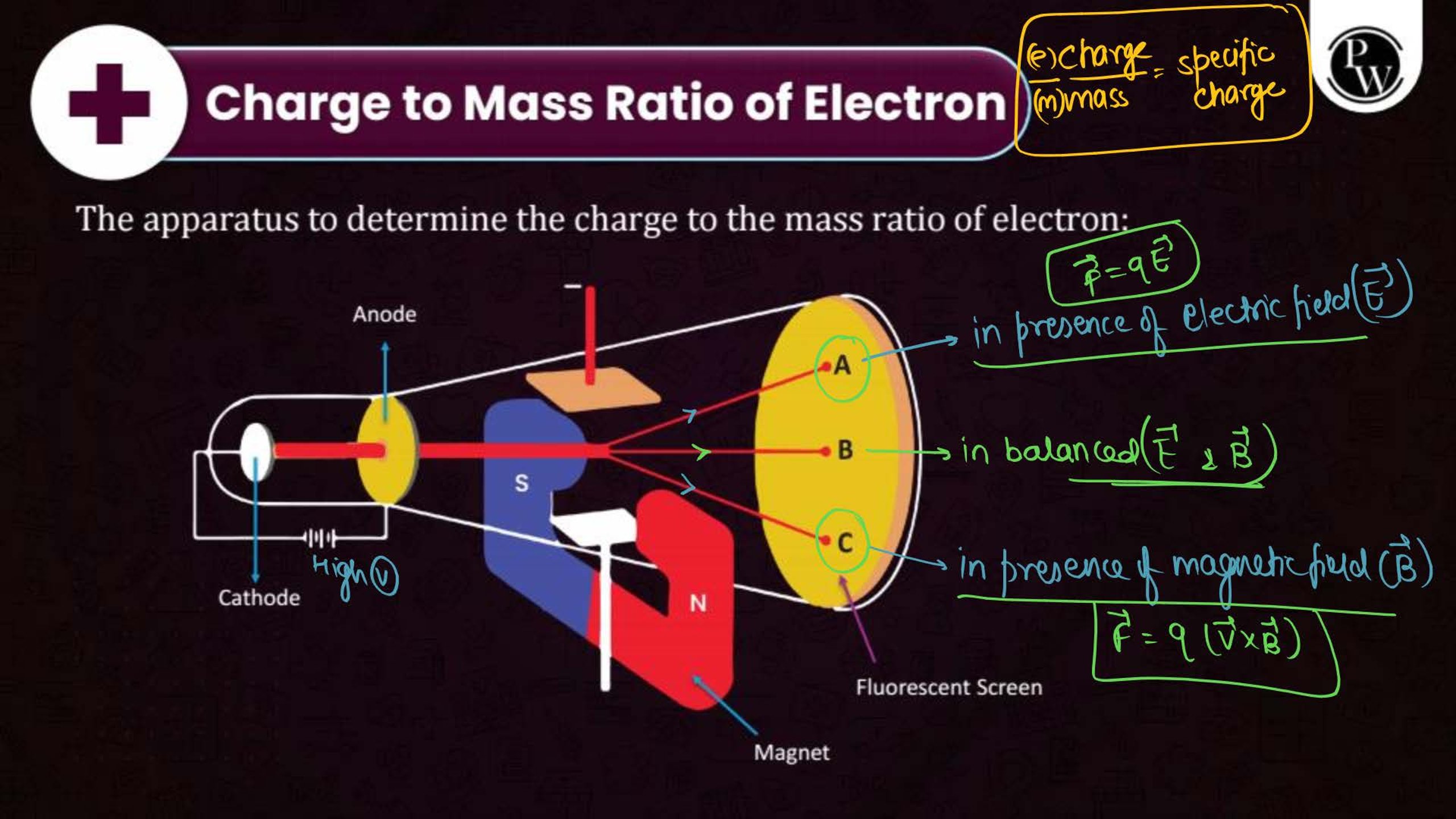
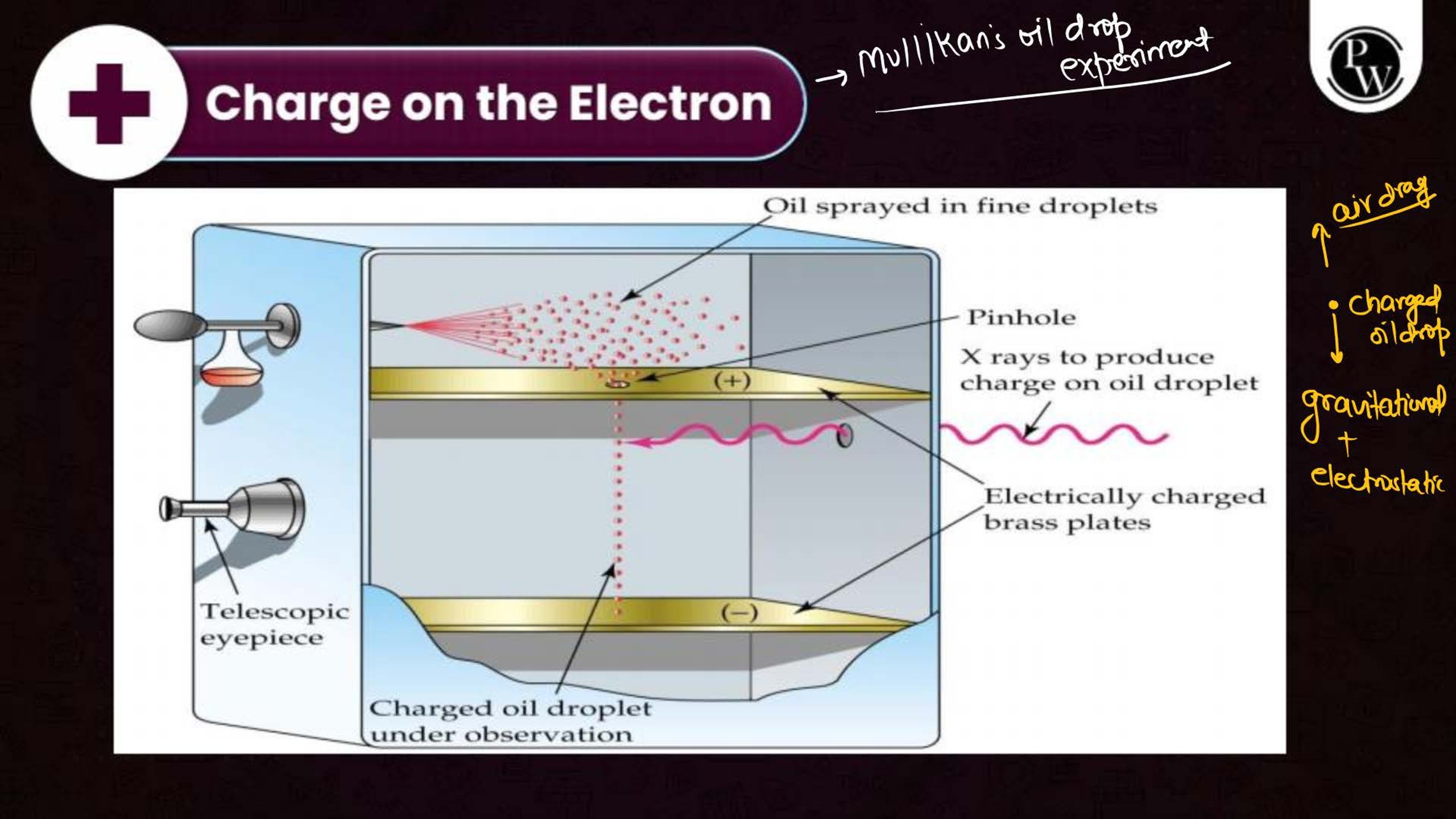
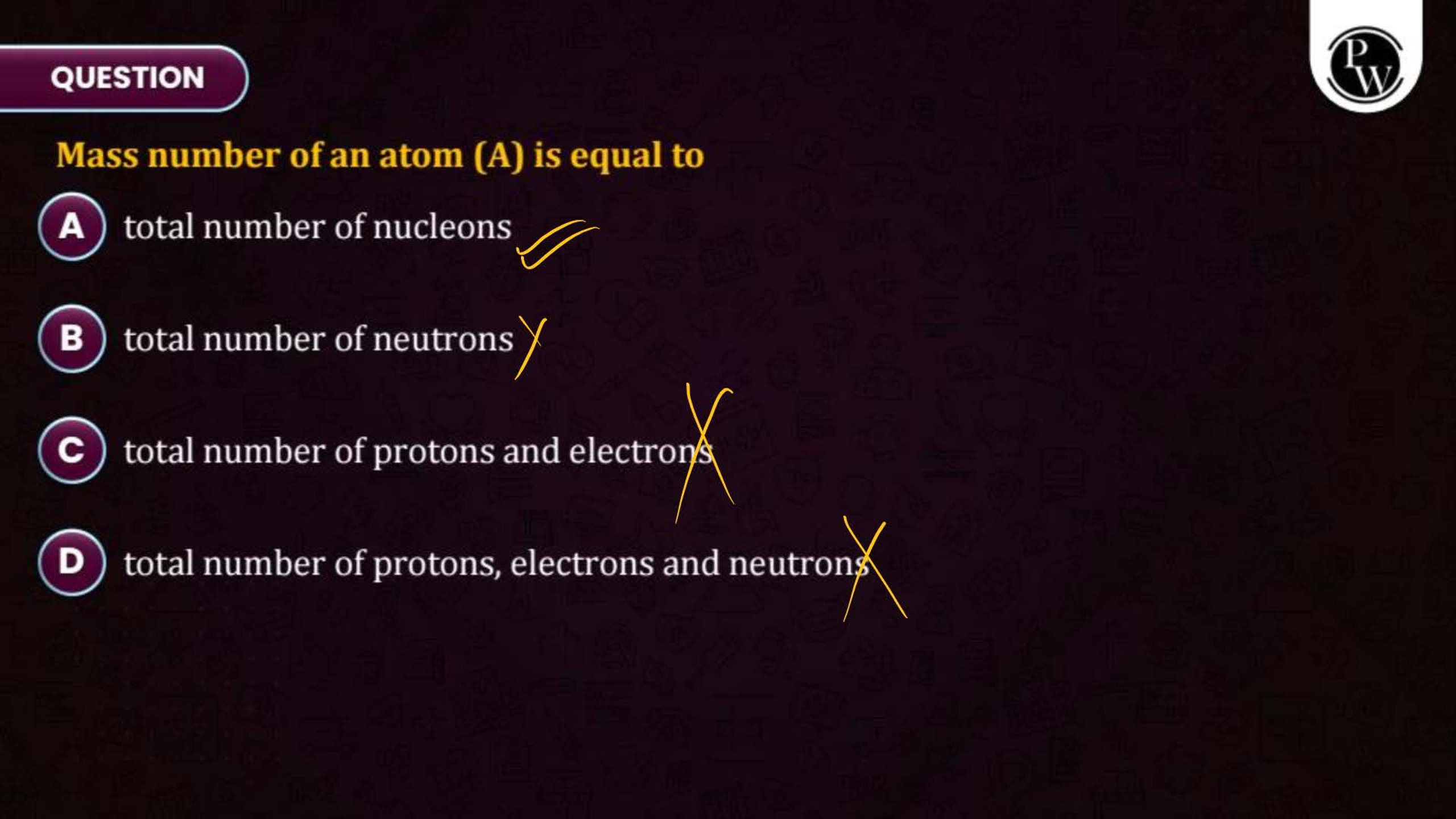
What is the Structure of an Atom?
Atoms are the smallest units of matter and consist of three primary subatomic particles: protons, neutrons, and electrons. The protons and neutrons form a dense core called the nucleus, located at the center of the atom. Surrounding the nucleus, electrons move in specific orbits or energy levels. These particles work together to determine the atom’s overall properties and behavior.
This section covers key historical and modern atomic models that describe the structure of an atom, including:
- J.J. Thomson’s Plum Pudding Model: Proposed that electrons are embedded in a positively charged "pudding" like structure.
- Rutherford’s Nuclear Model: Demonstrated the existence of a dense, positively charged nucleus at the atom's center.
- Bohr’s Model of the Atom: Introduced fixed energy levels or shells for electrons.
- Quantum Mechanical Model: Provides the most accurate depiction of atoms using probability distributions to locate electrons.
Weightage of Structure of Atom NEET Notes - Last 5 Years
The table below highlights the importance of this chapter in recent NEET exams (2021-2025):
| Weightage Following Previous Year’s Trends (2021-2025) | ||||||
| Chapter | Easy | Medium | Hard | Total | Average Q/yr | Weightage |
| Structure of an Atom | 2 | 3 | 3 | 8 | 1.60 | 3.04% |
The NEET 2026 syllabus suggests a weightage of 4% for this chapter in chemistry, emphasizing its importance.
| NEET Chapter Wise Weightage 2026 for Chemistry | |
| Topics | Weightage (%) |
| Structure of Atom | 4% |
Importance of Structure of Atom NEET Notes
Understanding the structure of an atom is vital for NEET preparation because it:
- Builds the foundation for advanced topics like chemical bonding and periodic classification.
- Helps in solving numerical problems involving atomic structure and quantum mechanics.
- Provides a deeper understanding of the behavior of elements and compounds.
Tips for the Structure of Atom NEET Notes
To excel in this chapter, students should:
- Focus on understanding atomic models and their limitations.
- Practice numerical problems, especially those involving quantum numbers and electron configurations.
- Revise diagrams and graphs frequently for better retention.
- Solve previous years’ NEET questions to identify patterns and frequently asked concepts.
Prepare for NEET with PW Online NEET Coaching ! Get comprehensive guidance with structured lessons, in-depth concepts, and interactive classes designed to help you succeed.
| NEET Exam Important Links | |
|---|---|
| NEET Syllabus | NEET Previous Year Question Papers |
| NEET Eligibility Criteria | NEET Exam Pattern |
Structure of Atom NEET Notes
Is the structure of an atom important for NEET?
Is Atomic Structure a tough chapter?
How many questions come from atomic structure in NEET?
What is the structure of an atom?










