
The structure and acetylene Formula, also known as ethyne. The chemical and structural formulas are given and explained in the following paragraphs. Acetylene is the simplest alkyne and is a hydrocarbon. Acetylene has two carbon atoms bound together by a triple bond, making it an unsaturated compound.
Acetylene Chemical Formula
Acetylene, also known as ethylene, is the simplest alkyne and hydrocarbon. Its chemical formula, C2H2, indicates that it contains carbon and hydrogen in a triple bond. This unsaturated compound is commonly used as a fuel and serves as a base for various other chemical compounds. Although pure acetylene is colorless and odorless, for practical purposes, it is often stored in liquid form due to handling difficulties.Also Read : Acetone Formula
The formula for acetylene with an extended formula of CHΞCH and a molar mass of 26.04 g mol-1. As the simplest alkyne, it contains triple bonds and is classified as a linear molecule (180 ºC). Its carbon atoms are hybridized sp, with two sp orbitals each; one bound to a hydrogen atom and the other forming a single bond with the other carbon atom. The remaining double bond, made up of two Π bonds, is created by four P orbitals without hybridization, which are perpendicular to the linear system. Common organic molecule representations can be used to depict its chemical structure.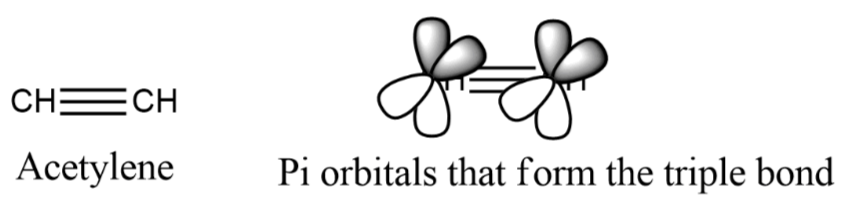
Formula of Ethyne
The ethyne formula includes both its chemical and structural representations, with the chemical name being C2H2. This name suggests that it is the simplest hydrocarbon, also known as an alkyne. Its unique characteristic is a triple bond between two carbon atoms, as shown in the ethene structural formula. This bond has a straight conformation at 180 degrees. The angle between the carbon atoms in this formula indicates their bond angle. The official IUPAC name for this compound is acetylene or ethyne.Preparation of Acetylene (Ethyne)
- Different preparative techniques can generate acetylene and is sometimes produced as a secondary result.
- In the past, it was a by-product of ethylene production via hydrocarbon cracking.
- The methods for producing acetylene include partial combustion of methane, refining during ethylene production, and hydrolysis of chemical carbide.
- It can also be created through the dehydrohalogenation of alkyl dihalides or vicinal dihalides.
Chemical Properties
The chemical properties of acetylene include the acetylene formula, molecular weight, bond angle, the reactions of the compound, the molecular mass of the compound, and the empirical formula of acetylene. Acetylene has the following chemical properties that give insight into its reactions, which provide insight into its chemical properties.- C2H2 is the formula for acetylene or its empirical formula
- It has a molecular weight of 26.04 g/mol.
- There are no hydrogen bonds between donors and acceptors
- Rotatable bonds are not present.
- The triple bond in the structural formula of ethyne indicates that they are unsaturated organic compounds.
- 180 degrees is the bond angle.
- Symmetrical compounds are chemical compounds.
Physical Properties Of Acetylene Formula
As defined by the molecular formula of acetylene, the physical properties of organic compounds are defined. An organic compound's physical properties include its melting point, boiling point, density, appearance, and crystal structure. The following are some of the physical properties.- At 760 mm Hg, the boiling point of the compound is -119 °F or -84.7 °C
- Gaseous organic compounds are generally found (pure)
- Gases such as acetylene are colourless
- The pure form of the compound has no smell, whereas the liquid form has a faint ether scent.
- As acetylene cannot exist in liquid form under natural atmospheric pressure, it has no melting point.
- At a minimum atmospheric pressure of 1.27atm, the melting point is 80.8°C.
- The compound is soluble in water, ethanol, carbon disulfide, acetone, benzene, and chloroform.
- Based on the ethyne molecular formula, the compound has a linear structure. Acetylene has a density of 1.1772 grams per liter or 1.1772 kilograms per cubic meter.
Acetylene Formula Uses
In addition to being used in fuels for a long time, acetylene is reportedly used in explosives. Here are some uses of acetylene.- It is used in welding.
- Used in portable lighting.
- Plastics and acrylic acid derivatives are made from it.
- Used in Radiocarbon dating
- The first natural semiconductor, polyacetylene, is produced from it.
Acetylene Formula FAQs
What is the chemical formula of acetylene?
The chemical formula of acetylene is C2H2.
What is the common name for acetylene?
Acetylene is commonly known as ethyne.
How is acetylene used in industry?
Due to its high flame temperature, acetylene is used as a fuel gas in welding and cutting torches. It's also a precursor in the synthesis of various organic chemicals.
Is acetylene highly flammable?
Yes, acetylene is highly flammable and can form explosive mixtures with air when not handled properly. It's usually stored and transported dissolved in acetone within specialized cylinders to reduce its explosive potential.
What is the structure of the acetylene molecule?
The acetylene molecule consists of two carbon atoms triple-bonded to each other (C≡C) and each bonded to a single hydrogen atom (H-C≡C-H). This linear structure gives acetylene its unique properties.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









