
Ammonium Sulfide Formula is (NH4)2S. Similarly, Ammonium Sulfide has a molar mass of 66.122 g mol-1. We see that the molecule forms with the help of one sulfur atom in which two ammonium cations NH4+ are attached. The chemical structure of the formula can be written as follows, based on the common organic molecule representations we use. Ammonium sulfide formula is a chemical compound with a unique formula that plays a significant role in various chemical processes.
Ammonium Sulfide Formula and Charge
The ammonium sulfide formula is (NH4)2S. This formula indicates that each molecule of ammonium sulfide is composed of two ammonium ions [(NH4)+] and one sulfide ion [(S)2-]. To better understand the charge distribution in the compound, let's break down the formula:
- The ammonium ion [(NH4)+] is a positively charged polyatomic ion with a charge of +1.
- The sulfide ion [(S)2-] is a negatively charged polyatomic ion with a charge of -2.
When two ammonium ions combine with one sulfide ion, the charges balance out, resulting in an electrically neutral compound.
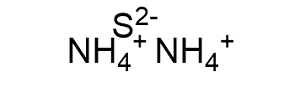
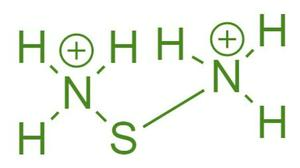
Ammonium Sulfide Formula: Ionic or Covalent?
Ammonium sulfide is an ionic compound. Ionic compounds are formed when atoms transfer electrons between each other to achieve a stable, full outer electron configuration. In the case of ammonium sulfide, the ammonium ions (NH4+) lose one electron each to become positively charged, while the sulfide ion (S2-) gains two electrons to become negatively charged. This charge difference creates electrostatic attraction between the ions, holding them together in a crystal lattice structure.
Ammonium Sulfate Formula
Ammonium sulfate is another important chemical compound that contains the ammonium ion. Its chemical formula is (NH4)2SO4. In this compound, two ammonium ions [(NH4)+] are combined with one sulfate ion [(SO4)2-]. Ammonium sulfate is commonly used as a fertilizer and in various industrial applications.
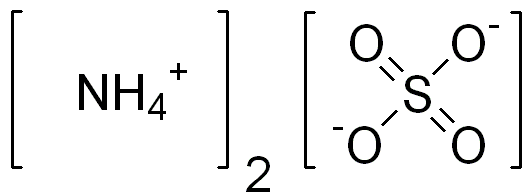
Ammonium Sulfite Formula
Ammonium sulfite, on the other hand, has the chemical formula (NH4)2SO3. This compound consists of two ammonium ions [(NH4)+] and one sulfite ion [(SO3)2-]. Ammonium sulfite is used in the treatment of paper and textiles.
Ammonium Sulfate Formula: Ionic Compound
Ammonium sulfate, like ammonium sulfide, is an ionic compound. It is formed by the combination of positively charged ammonium ions and negatively charged sulfate ions. The electrostatic forces between these ions result in the solid, crystalline structure of ammonium sulfate.
Ammonium Sulfate Formula Mass
To calculate the mass of the ammonium sulfate formula, you must add the atomic masses of all the atoms in one formula unit. The formula mass of (NH4)2SO4 can be calculated as follows:
Atomic mass of N (nitrogen) = 14.01 g/mol
- Atomic mass of H (hydrogen) = 1.01 g/mol
- Atomic mass of S (sulfur) = 32.07 g/mol
- Atomic mass of O (oxygen) = 16.00 g/mol
Now, calculate the formula mass:
(2 x 14.01 g/mol) + (8 x 1.01 g/mol) + 32.07 g/mol + (4 x 16.00 g/mol) = 28.02 g/mol + 8.08 g/mol + 32.07 g/mol + 64.00 g/mol = 132.17 g/mol
The formula mass of ammonium sulfate is approximately 132.17 grams per mole.
Physical Properties of Ammonium Sulfide
The yellow-orange compound of Ammonium Sulfide formula exists as a crystalline solid at temperatures below -18 ºC. As for its scent, it emits an unpleasant odor reminiscent of both rotten eggs and ammonia. Its melting point is 0 °C and boiling point is 40 °C, with a density of 1 g mL-1. It is soluble in water and ethanol, but insoluble in toluene, benzene, hexane, and ether. However, caution should be taken as exposure to temperatures above 0 ºC becomes unstable.
| Related Links | |
| Aluminium chloride formula | Electronic configuration of copper |
| Aluminium fluoride formula | Aluminium Iodide Formula |
Ammonium Sulfide FormulaFAQs
What is the mass of ammonium sulfate in AMU?
What is the mass of NH2SO4?
What is the molar mass of NH4S2?










