
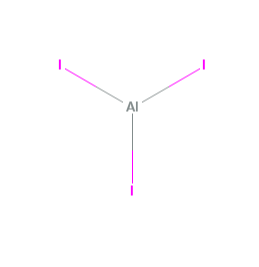
Aluminium Iodide formula is AlI₃ is a compound composed of aluminium (Al) and iodine (I) atoms. It is an ionic compound formed by transferring electrons between aluminium and iodine, forming charged ions.
There are two elements in the Aluminum Iodide Formula: iodine and aluminium. It is an ionic molecule that catalyzes organic processes. It is also possible to create hexahydrates by mixing hydrogen iodine or hydriodic acid with metallic aluminium or aluminium hydroxide. It is a Lewis acid that absorbs moisture from the air, breaking down aryl ethers and deoxygenating epoxides.
Aluminium Iodide Formula
Aluminium iodide is also known as aluminium triiodide. Its chemical formula, AlI₃, indicates that it consists of one aluminium atom and three iodine atoms. The subscript "3" in the formula signifies that there are three iodine atoms for every one aluminium atom in the compound.
Also Read: Methane formula
Aluminum Iodide Formula and Structure
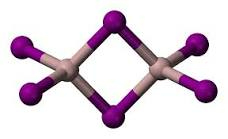
Aluminium iodide formula is AlI3. It is dimeric (meaning it is composed of two identical simpler molecules), Al2I6, similar to AlBr3. Its gas phase characterizes the monomeric and dimeric forms. In addition, the monomer AlI3 is a triangular planar, and its bridged dimer Al2I6 is a triangular planar like Al2CL6 and Al2Br6. A dimer with equilibrium geometry may also be referred to as floppy.
As with aluminium bromide, aluminium iodide has the chemical formula AlI3. It is dimeric (meaning it is composed of two identical simpler molecules), Al2I6, the same as AlBr3. The gas phase characterizes the monomeric and dimeric forms of its structure. The monomer, AlI3, is a trigonal planar, and the bridged dimer, Al2I6, has an equilibrium geometry similar to Al2CL6 and Al2Br6. The dimer can also be referred to as floppy.
Also Read: Lead Acetate Formula
Aluminium Iodide Formula Compound
The aluminium iodide formula is a chemical compound that forms when aluminium and iodine react together. It is an ionic compound in which aluminium, a metal, donates three electrons to each iodine atom, a non-metal. This electron transfer leads to the formation of Al³⁺ cations and I⁻ anions, resulting in the stable ionic compound AlI₃.
Aluminium Iodide Equation
The formation of the aluminium iodide formula can be represented through a chemical equation, typically depicted as:
2Al + 3I₂ → 2AlI₃
In this equation, two aluminium atoms react with three molecules of iodine (I₂) to yield two molecules of aluminium iodide (AlI₃). The balanced equation demonstrates the reaction's stoichiometry, showing that two moles of aluminium react with three moles of iodine to produce two moles of aluminium iodide.
Aluminium Iodide Chemical Formula
The chemical formula for aluminium iodide, AlI₃, summarizes the composition and ratio of elements within the compound. It is important to note that aluminium iodide is a solid ionic compound with a distinct crystal lattice structure, and it is used in various chemical reactions and synthesis processes in chemistry and industry.
Also Read: Magnesium Iodide formula
Aluminum Iodide Physical Properties
As a colourless powder, it has a density of 3.98 g/cm3, a melting point of 189.4oC, a boiling point of 360oC, and a molar mass of 407.69 g/mol. It can dissolve in water, ethanol, diethyl ether, carbon disulfide, pyridine, and sulfur dioxide.
Aluminum Iodide Chemical Properties
It is an ionic compound applied as a spray to treat animal stalls and added as a catalyst (a substance added to speed up a reaction) in some organic reactions. Since it releases significant heat and is exothermic, scientists and businesses conduct it under a special heat-resistant container and fume hood. In water, iodine reacts with the aluminium iodide formula, generating a purple vapour.
Aluminum Iodide Uses
It is commonly used as an animal stall cleaner, where its vapour kills bacteria that can cause respiratory diseases. It is used in organic chemistry sometimes as a catalyst that accelerates reactions between elements and compounds. In addition, it breaks carbon-oxygen bonds and nitrogen-oxygen bonds, removing oxygen from epoxides.
| Related Links | |
| Potassium Acetate formula | Nickel Chloride formula |
| Porpionic Acid formula | Hyponitrous Acid Formula |
Aluminium Iodide Formula FAQs
Is aluminum iodide a compound?
What is AlI₃ called?
What is the compound formed by aluminium and iodine?
How do you write aluminum iodide?










