
Argon Gas Formula , or Ar with atomic number 18, is a noble gas used in various applications. Monatomic argon is commonly found in fluorescent tubes. Its molecular formula is simply Ar. This gas has a higher density than air and is non-combustible, colourless, and odourless. However, high heat or fire exposure can cause it to rupture violently. Similarly, when liquid argon comes into contact with icy water, it can create intense boiling. This makes it an essential gas for food packaging purposes.
Argon Gas Formula Mass
The formula mass of argon is determined by calculating the atomic mass of a single atom and then multiplying it by the number of atoms in one mole. For argon, its atomic mass is approximately 39.95 atomic mass units (amu). Therefore, the formula mass of argon (Ar) is also approximately 39.95 amu.
Argon Oxide Formula
Argon does not readily form compounds, including oxides. Unlike some other elements, argon remains chemically inert, so it doesn't readily combine with other elements to form compounds like oxides.
Argon Gas Uses
Argon gas has various practical applications due to its unique characteristics. Some of its primary uses include:
1. Welding: Argon is often used as a shielding gas in welding processes, such as TIG (Tungsten Inert Gas) welding, to protect the molten metal from reacting with the surrounding air.
2. Light Bulbs: Argon is employed in some types of electric light bulbs, providing a stable and inert atmosphere inside the bulb.
3. Laboratory and Scientific Applications : Argon is used as a carrier gas in gas chromatography and as a background gas in certain spectroscopic techniques.
4. Cryogenics: In its liquid form, argon is used in cryogenic applications to achieve extremely low temperatures.
5. Preservation: Argon is used to preserve and protect valuable documents, artwork, and historical artefacts by creating an inert environment that prevents deterioration.
Also Read: Formic Acid Formula
Helium Gas Formula
Helium, another noble gas, has the chemical symbol He. It is atomic number 2 on the periodic table and is known for its low density and inert properties. Helium is often used in applications such as filling balloons and cooling in scientific research.
Argon Chemical Formula and Physical State
The chemical formula of argon is Ar, and it exists as a colourless, odourless, and tasteless gas under normal conditions (standard temperature and pressure). Argon is the most abundant noble gas in the Earth's atmosphere after nitrogen and oxygen.
Also Read: Silver Acetate formula
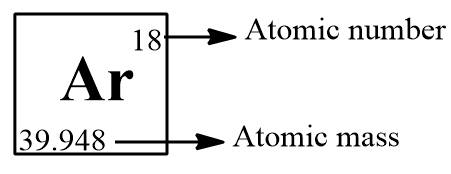
Argon Atomic Number
The atomic number of argon is 18. It represents the number of protons in the nucleus of an argon atom, distinguishing it from other elements on the periodic table.
Argon Atomic Mass
The atomic mass of argon (Ar) is approximately 39.95 atomic mass units (AMU). This value represents the average mass of an argon atom, considering the isotopic composition of naturally occurring argon.
Also Read: Glucose Chemical Formula
5 Uses of Argon
1. Welding: As mentioned earlier, argon is commonly used as a shielding gas in welding to prevent the oxidation and contamination of welds.
2. Lighting: In various types of light bulbs, argon is used to prolong the filament's life by reducing evaporation.
3. Laboratory and Analytical Techniques: Argon is a carrier gas in gas chromatography and is used in some spectroscopic methods for sample analysis.
4. Cryogenic Applications: Liquid argon is used in cryogenics to reach ultra-low temperatures for research and cooling purposes.
5. Conservation: Argon is employed to preserve and protect sensitive historical artefacts and documents by creating a stable, inert environment that prevents decay.
Argon's diverse applications make it valuable in various industries and scientific fields despite its natural inertness and lack of compound formation.
| Related Links | |
| Aluminium chloride formula | Lead Acetate Formula |
| Aluminium fluoride formula | Lead Iodide Formula |
Argon Gas Formula FAQs
Is argon gas Ar or Ar2?
What is argon as a gas?
What is the general formula of argon?
How is argon gas made?










