
Formic Acid Formula is also referred to as Formylic acid or Methanoic acid. Formic acid is the simplest carboxylic acid, containing a single carboxylic acid group (COOH) attached to a hydrogen atom. The Formic Acid Formula is HCOOH or HCO 2 H.
It is a colorless liquid with a pungent odor and a sour taste. It is corrosive and miscible in acetone, methanol, ether, ethyl acetate, and ether. Benzene, xylenes, and toluene are partially soluble in it. Several insects, including bees and ants, naturally produce it. Beekeepers use it as a miticide because it is widely used in livestock feed as a preservative and antibacterial agent.Formic Acid Formula
There is a pungent acrid odor to formic acid, which is systematically named as methanoic acid and is also known as carboxylic acid. The Latin or Greek names of its source gave these names to it. The salts of formic acid are mildly irritating to the eyes. They are corrosive in nature and skin sensitizers. Other names include methanoic acid, formylic acid, and ammonic acid. Also Read :Formic Acid Formula Structure
Formic acid formula structure is the simplest carboxylic acid. It consists of a hydrogen atom joined by a carboxylic acid group. Hydrogen’s small size does not interfere with the bond angles between the O-C-O groups in carboxylic acids. The O-C-O bond angle is 116.8 degrees. The incoming hydrogen atom forms a bond with the hydroxyl Oxygen. The dihedral angle of the H-C-O-O bond is 180 degrees.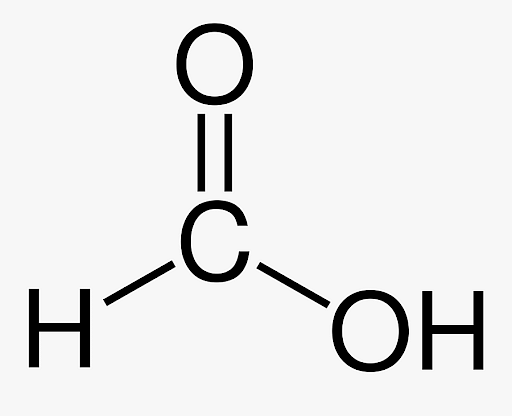
Properties Of Formic Acid (Methanoic Acid)
Formic acid has Following Properties :- HCOOH is the chemical formula for formic acid. This acid is also called methanoic acid, formylic acid, and aminic acid.
- Formic acid has a molecular weight of 46.025 grams per mole.
- Formic acid has a density of 1.220 grams per liter.
- It has a melting point of 8.4°C and a boiling point of 100.8°C.
- There is one covalently bonded unit and two hydrogen bonds, an acceptor.
- In addition to being miscible in water, formic acid is miscible in acetone, ether, methanol, ethanol, and ethyl acetate. However, formic acid is partially soluble in benzene, toluene, and xylene. Insects, bees, and ants naturally produce formic acid.
- Despite not being used as a solvent, it is a protic solvent with a high acidity.
- As well as being corrosive and skin sensitizers, formic acid is sometimes mildly irritating to the eyes.
- In reaction with phosphoric pentachloride, formic acid forms hydrogen chloride, formyl chloride, and phosphoryl chloride.
- The chemical formic acid is found in some ant species, venom, and even in the secretion of stinging nettles.
- In high concentrations, it can be very harmful, but in low concentrations, it is very effective.
- Due to its antibacterial properties, it is used as a food preservative.
Also Read: Propan-2-ol Formula
Chemical Properties of Formic Acid
As a carboxylic acid with some aldehyde-like properties, it reacts easily with alcohol to form esters. Formic acid breaks down into carbon monoxide and water in the presence of acids or heat. Platinum decomposes it into carbon dioxide and hydrogen in the presence of platinum.Natural Occurrence of Formic Acid
Formic Acid is found in nature. When bees and ants sting, formic Acid reacts with our skin, causing pain. Formic Acid can activate our pain receptors, as well as have antibacterial properties. Therefore, Formic Acid is widely used for a variety of purposes.Also Check – Aluminium Nitrate Formula
Uses of Formic Acid
Among the many uses of Formic Acid are:- In combination with citric acid, formic acid removes iron oxide deposits more effectively than when used alone.
- The use of formic acid in saturated monocarboxylic acids is one of its major applications.
- As a reducing agent, formic acid is very useful for reducing potassium dichromate and sodium dichromate.
- Formic acid is used in tanning and dyeing industries, but other cheaper assets are available, so formic acid is used less or only in cases where it is advantageous.
- Due to its antibacterial properties, formic acid is highly used in agriculture.
- Known as a pesticide, it is proven beneficial in preventing crops from being attacked by different pests.
Formic Acid Tests
The standard tests for carboxylic acids include:- Changing the color of litmus paper from blue to red, producing carbon dioxide when mixed with a saturated solution of NaHCO 3 ,
- Forming a silver mirror upon heating with ammonium silver nitrate, and generating a crimson cuprous oxide precipitate when heated with Fehling's solution.
- Additionally, heating with mercuric chloride results in the production of mercurous chloride and neutralization with ammonium hydroxide followed by the addition of ferric chloride solution creates a red color.
- Concentrated sulfuric acid also causes a release of carbon monoxide gas upon heating, which burns with flame.
Also Check – Ammonium Nitrate Formula
Interesting Facts of Formic Acid Formula
- Formic acid formula, also known as methanoic acid according to IUPAC, is a colorless and flammable liquid with a pungent odor that can cause discomfort when inhaled or comes into contact with skin.
- When carbon monoxide and sodium hydroxide combine with water, they produce sodium formate which then reacts with sulphuric acid to create formic acid.
- It belongs to the carboxylic acids family and is the first compound in its series. Its distinctively harsh and unpleasant smell is water-soluble and miscible.
- Heating formic acid with Fehling's solution produces a crimson cuprous oxide precipitate, while heating it with mercuric chloride solution results in the formation of a white precipitate of mercurous chloride.
- In the industrial world, saturated monocarboxylic acids are greatly improved by the addition of formic acid.
Formic Acid Formula FAQs
What is the Chemical Name for Formic Acid?
The chemical name of formic acid is "methanoic acid." Its molecular formula is HCOOH, consisting of one carbon atom (C), one oxygen atom (O), and two hydrogen atoms (H).
Why is HCOOH called formic acid?
The name "formic acid" was coined by the English chemist and naturalist Joseph Priestley in 1776 when he isolated the acid from red ants (Formicidae family). The pungent odor of formic acid resembles the smell of ant venom, which is why it was associated with ants, and thus, the name "formic acid" was given to this compound. The name reflects its historical origin and the source from which it was first isolated.
Is formic acid and methanoic acid the same?
Formic acid is also known as "methanoic acid." These two names refer to the same compound. "Formic acid" is the common name that is more widely used, while "methanoic acid" is the systematic IUPAC (International Union of Pure and Applied Chemistry) name. So, whether you call it "formic acid" or "methanoic acid," you refer to the same chemical compound with the molecular formula HCOOH.
What state is Formic Acid?
In its liquid state, formic acid is a colorless and pungent-smelling liquid. It has a boiling point of approximately 100.8 degrees Celsius (213.4 degrees Fahrenheit) and a melting point of around 8.4 degrees Celsius (47.1 degrees Fahrenheit). Due to its relatively low boiling point can also be present as a gas when heated or under certain conditions.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









