
Lead acetate formula is Pb(CH₃COO)₂, also known as lead(II) acetate. It is a white crystalline solid soluble in water and has a sweetish taste. Lead acetate is a toxic substance and should be handled with extreme care due to its harmful effects on human health and the environment. Lead acetate can be prepared by reacting acetic acid (CH₃COOH) with lead oxide (PbO). The reaction results in the formation of lead acetate and water.
Lead Acetate Formula
The lead acetate formula is Pb(CH₃COO)₂, also written as Pb(CH₃COO)₂•3H₂O when accounting for water molecules present in the compound. This formula breaks down as follows: - Pb: Represents the chemical symbol for lead, denoting the presence of one lead (Pb) atom. - (CH₃COO)₂: Indicates the acetate ion, which consists of two acetate (CH₃COO-) groups. Each acetate group comprises carbon (C), hydrogen (H), and oxygen (O) atoms, bonded together. - H₂O: Refers to three water (H₂O) molecules associated with the lead acetate compound.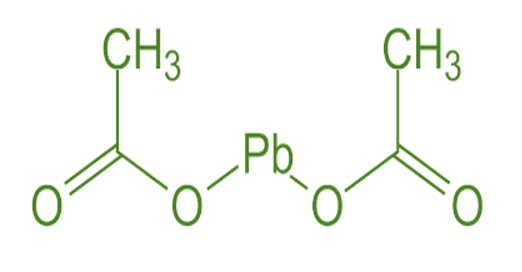
Also Read: Acetone Formula
Lead Acetate Formula Structure
The structure of lead acetate formula can be described as a coordination compound where the lead ion (Pb²⁺) is coordinated with two acetate ions (CH₃COO-) and three water molecules (H₂O). The acetate ions bond to the lead ion through their oxygen atoms, forming coordination bonds. This compound often appears in the form of colorless or white crystals, and its structure is vital in understanding its chemical behavior and reactivity.Also Read: Acetamide Formula
Lead Acetate Formula Molecular Weight
To determine the molecular weight of lead acetate formula, one needs to sum the atomic masses of all the constituent elements and molecules within the formula. Here's how to calculate it: - Pb: The atomic mass of lead (Pb) is approximately 207.2 g/mol. - (CH₃COO)₂: To find the molecular weight of the acetate ion, you would sum the atomic masses of its constituent elements (C, H, and O). The molar mass of acetate is approximately 59 g/mol. - 3H₂O: Water (H₂O) has a molar mass of approximately 18 g/mol. Now, we can calculate the molecular weight of lead acetate: Molecular weight of Pb(CH₃COO)₂•3H₂O = (1 Pb atom × 207.2 g/mol) + (2 acetate ions × 59 g/mol) + (3 water molecules × 18 g/mol) ≈ 382.2 g/mol.Also Read: Acetylene Formula
Lead Acetate Formula Molar Mass
The molar mass of lead acetate is approximately 382.2 grams per mole (g/mol). This value is significant in various chemical calculations, such as determining the amount of lead acetate in a given sample, understanding its behavior in chemical reactions, and performing stoichiometric calculations.Properties of Lead Acetate
Lead acetate, also known as lead(II) acetate, is a toxic chemical compound with a few notable properties. Handling lead acetate with great care is essential due to its toxicity. Here are some of its key properties| Properties of Lead Acetate | |
| Name | Lead Acetate |
| Also Known as | Plumbous Acetate, Salt Of Saturn, Goulard’s Powder, lead (II) ethanoate |
| Appearance | Colourless Efflorescent Crystals or white powder |
| Chemical Formula | Pb(C 2 H 3 O 2 ) 2 |
| Melting Point | 280 °C (anhydrous) 75 °C (trihydrate) 22 °C (decahydrate) |
| Boiling Point | Decomposes |
| Density | 3.25 g/cm³ (anhydrous) 2..55 g/cm 3 (trihydrate) 1.69 g/cm 3 (decahydrate) |
| Molar Mass | 325.29 g/mol (anhydrous) 379.33g/mol (trihydrate) |
| Solubility in Water | Soluble in water |
Uses of Lead Acetate
Lead acetate was once popular as a sweetener, thanks to its mildly sweet taste. The Romans would boil grape juice in lead pots to produce a reduced sugar syrup called defrutum. However, it was later discovered that this practice was causing lead poisoning due to the toxic nature of lead compounds, including lead acetate. As a result, regulations were put in place to ban the use of lead acetate as a sweetener. Despite its previous widespread use in the cosmetic industry, current restrictions have greatly limited its presence in products, with hair coloring being one of the few remaining uses. In fact, many areas have completely banned it from food and cosmetic items due to its harmful effects on health and reproduction. Lead acetate solution, also known as Goulard's Extract, has found a place in modern medicine as an astringent for constricting mucous membranes and exposed tissues. French surgeon Thomas Goulard first introduced this solution made up of lead acetate and lead oxide. Paper containing lead acetate has been used to detect poisonous gas H2S by reacting with hydrogen sulfide gas to form lead sulfide when moist. Additionally, it has been utilized for cleaning and maintaining stainless steel suppressors and compensators.| Related Links | |
| Phthalic Acid formula | Galactose Formula |
| Pentane formula | Glucose Chemical Formula |
Lead Acetate
How do you write lead acetate ?
Lead acetate is typically written as "Pb(CH₃COO)₂" or "Pb(CH₃COO)₂•xH₂O," where "Pb" represents the chemical symbol for lead, and "(CH₃COO)₂" denotes two acetate ions. The "xH₂O" represents water molecules that may be associated with the compound.
What is the chemical name of Pb(CH₃COO)₂?
The chemical name of Pb(CH₃COO)₂ is lead(II) acetate or lead diacetate.
What is the formula for lead acetate in Class 12?
In a chemistry context, particularly in Class 12 or higher-level chemistry studies, the formula for lead acetate is still Pb(CH₃COO)₂. This formula represents the composition of lead acetate, which consists of lead ions and acetate ions.
What is the formula of PbO?
The chemical formula of lead(II) oxide is PbO. It represents the combination of one lead (Pb) atom and one oxygen (O) atom. Lead(II) oxide is a compound with industrial applications, primarily as a component in various types of glass, ceramics, and pigments.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









