
Butyric acid , with a chemical formula of C 3 H 7 COOH, is a type of carboxylic acid. It is also referred to as Butanoic acid, Propyl Formic acid, or Ethyl Acetic acid. While not commonly found in nature, its esters are present in plant oils and animal fats. A fatty acid is another type of carboxylic acid that has a long aliphatic chain and can be saturated or unsaturated. This colorless, oily liquid has an unpleasant odor reminiscent of vomit or body odor. 1814 French chemist Michel Eugene Chevreul was the first to observe impure butanoic acid. As a food acidity regulator, butanoic acid acts as both an ammonium salt and an acetate salt with four carbons.
What is the Butanoic Acid Formula?
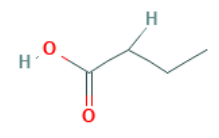
Butanoic acid, also known as butyric acid, has the IUPAC nomenclature CH 3 CH 2 CH 2 COOH and can be alternatively represented as C 4 H 8 O 2 . This formula contains a single double bond between the carbon and oxygen atoms of the carboxylic acid group. Butyric acid is a straight-chain compound with four carbon atoms. It is commonly referred to as butyrates or simply the butyrates.
Butyric Acid Structural Formula
There is only one double bond in the carboxylic group of butyric acid, which defines it as the short-chain linear structure of the alkyl carboxylic acid. Its structural formula is given below.
Properties of the Butyric Acid
Chemical compounds each possess unique properties that are used for identification. The physical attributes of butyric acid include being a colorless liquid and emitting an unpleasant odor during hydrolysis. With a molecular weight of 88.106 g/mol, it has a recorded density of 1.135 g/cm3 at -43 °C and 0.9528 g/cm3 at 25 °C. Its melting point is -5.1 °C (or 22.8 °F or 268.0 K) and it has a monoclinic crystal structure. The compound's boiling point is 163.75 °C (converted to 326.75 °F or 436.90 K). While insoluble in water, it can be dissolved in ethanol, ether, and is slightly soluble in tetrachloromethane (CCl 4 ).
Also Check – Aluminium Nitrate Formula
Production of Butyric Acid
Both industrial methods and microbial biosynthesis can be used to produce butyric acid. In the industrial production of butyric acid, the butyraldehyde is oxidized. The butanal is also known as butyraldehyde. A two-step chemical reaction is responsible for producing butyric acid.
H 2 + CO + CH 3 CH = CH 2 → CH 3 CH 2 CH 2 CHO
CH 3 CH 2 CH 2 CHO + O 2 → CH 3 CH 2 COOH
It is not self-catalyzed; instead, a catalyst is required. Calcium chloride salt is used to separate the end product.
Also Check – PH of Weak Acid Formula
Natural Sources of Butyric Acid
The natural source of butyric acid includes the following compounds
- Plant Oils,
- Butter,
- Animal Fat,
- Breast Milk,
- Bovine Milk, and
- Parmesan Cheese.
Also Check – Acids and Bases Formula: Types of Reaction
Salts and Esters of Butanoic Acid
At physiological pH, butyric acid is also found as salt and ester. The salts are also referred to as butyrates or butanoates. The butyric acid formula is CH 3 CH 2 CH 2 COOH. It can be defined as a conjugate base of butyric acid. As an example of a butyric acid salt, sodium butyrate is an example of a butyric acid ester.
- Butyl butyrate
- Butyryl-CoA
- Cellulose acetate butyrate.
- Estradiol benzoate butyrate.
Uses of Butyric Acid
Butyric acid serves a variety of purposes, particularly in the production of butyrates, which are esters derived from butyric acid. These compounds have multiple applications, such as being used to create esters like butyl butyrate. Another common use for butyric acid is in the production of cellulose acetate butyrate (CAB), which is often found in paints due to its durability. This acid also plays a crucial role in plastic production through its anhydride form. Additionally, it serves as a flavoring agent in certain products.
Butyric Acid Formula FAQs
Q1. What is the chemical formula of butyric acid?
Q2. What is the common name for butyric acid?
Q3. What gives butyric acid its distinctive odor?
Q4. Is butyric acid found naturally in certain foods?
Q5. What are some industrial uses of butyric acid?










