
Calcium Chloride Formula: Calcium chloride formula is CaCl 2 . It is an inorganic salt. This substance presents itself as a white crystalline solid at room temperature and exhibits exceptional solubility in water. Its synthesis involves the neutralization of hydrochloric acid with calcium hydroxide.
In various forms, calcium chloride is encountered as a hydrated solid, with a general Calcium Chloride formula of CaCl 2 ·nH 2 O, where n can take on values of 0, 1, 2, 4, and 6. These compounds are primarily used for de-icing and dust control purposes. The anhydrous salt, due to its hygroscopic and deliquescent properties, also finds use as a desiccant.
It's an ionic compound. calcium chloride formula is CaCl 2 and goes by various names like calcium chloride anhydrous or calcium dichloride. Essentially, it's composed of calcium and chlorine, appearing as a crystalline solid that's white at room temperature. Calcium chloride exhibits high solubility in water, making it hygroscopic. Notably, its enthalpy change during the dissolution process is remarkably high, and it lacks any distinctive odor. This chemical is frequently used for tasks such as dust control and de-icing.
Calcium Chloride Formula Structure
Calcium chloride formula is CaCl 2 . In the composition of calcium chloride molecules, a single calcium cation and two chloride anions establish two ionic connections. The schematic representation of calcium chloride's molecular structure is displayed to the right. Notably, the calcium cation carries a charge of +2, while each chloride anion bears a -1 charge. Consequently, the overall molecule maintains a neutral electric charge.
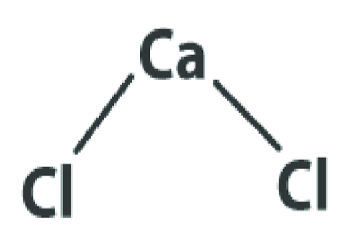
Calcium Chloride Formula Properties
- Chemical Formula: Calcium Chloride Formula is CaCl 2.
- Molecular Weight: 110.98 g/mol
- Density (Anhydrous): 2.15 g/cm3
- Melting Point (Anhydrous): 772–775 °C
- Boiling Point (Anhydrous): 1,935 °C
Production of Calcium Chloride
To produce calcium chloride, the following steps can be followed:
Begin by donning gloves and placing limestone into a beaker until it's filled to one-fourth of its original volume.
Add approximately one-fourth of a beaker's capacity of hydrochloric acid (HCl) to the limestone. As the hydrochloric acid interacts with the limestone, bubbles will emerge.
Gently stir the contents in the beaker, monitoring the reaction until it reaches completion. If the limestone has entirely dissolved, consider adding a small amount of additional limestone.
After the solution ceases bubbling, filter out the particulate matter by passing it through filter paper.
Proceed to heat the calcium chloride solution in a separate beaker. When the water evaporates, solid calcium chloride will remain as the end product.
Calcium Chloride Solutions and Their Applications
Calcium chloride is valued for its remarkable solubility in water, making it a popular choice for creating high-density solutions. Industries like oil and gas drilling leverage this solubility when working on well completions or reworks.
Studies have determined the densities of calcium chloride solutions at various concentrations and temperatures. The viscosity of these solutions holds particular importance in engineering design and the practical use of such solutions in porous media flow.
During the 1980s, extensive research focused on the thermodynamics of calcium chloride solutions with a primary aim to validate and improve the Pitzer equations, essential for calculating activity coefficients and other parameters in high-ionic-strength electrolyte solutions.Commercially, calcium chloride is produced through diverse processes. These methods include refining natural brines, the reaction of calcium hydroxide with ammonium chloride in the manufacturing of Solvay soda ash, and the reaction of hydrochloric acid with calcium carbonate.
Another noteworthy property of calcium chloride is its moisture-absorbing ability when exposed to the air. This hygroscopic feature is especially harnessed in the case of anhydrous calcium chloride, widely used as a desiccant or drying agent.
Uses of Calcium Chloride
Calcium chloride finds diverse uses across multiple industries and settings:
Industrial Enhancements: It is a common additive in plastics, blast furnaces, and wastewater treatment plants to enhance processes and properties.
De-Icing Agent: Calcium chloride serves as a de-icing agent, effectively lowering the freezing point of water. This property makes it crucial for preventing ice formation on road surfaces.
Dehumidifiers: In both domestic and industrial settings, it is used in dehumidifiers to reduce moisture levels in the air.
Food Industry: Calcium chloride is utilized in the food industry, where typical daily consumption ranges from 160 to 345mg. It's used in various applications, such as adding bioavailable calcium to calcium carbonate-shelled creatures in aquariums and creating canned vegetables or fruit juice caviar alternatives. It also functions as an electrolyte in sports drinks and other beverages, including water.
Flavoring: In the culinary world, it's used to impart a salty flavor to pickles, and it finds applications in a range of food processing, from beer brewing to cheesemaking.
In sum, calcium chloride is a versatile compound with a wide range of applications, from industrial processes to food production and even maintaining safe road conditions during icy weather.
| Related Links | |
| Potassium Fluoride Formula | Potassium Chlorate Formula |
| Potassium Bromate Formula | Gold Formula |
Calcium Chloride Formula FAQs
What is the chemical formula for calcium chloride?
What does calcium chloride look like at room temperature?
Is calcium chloride soluble in water?
How is calcium chloride produced?
Why is calcium chloride used as a desiccant?










