
Dalton's Law Formula represent the characteristics of gas behavior, particularly in gas mixtures. It was initially presented by English chemist John Dalton in 1802. This law asserts that in a mixture of non-reactive gases, the total pressure is the sum of the partial pressures of each gas. This principle is also known as Dalton's Law of Partial Pressures.
Dalton's Law Formula
In a mixture of non-reactive gases, the total pressure equals the sum of each gas's partial pressures. Additionally, this law has a connection to the ideal gas law.
The total pressure for a mixture of n gases can be expressed as:
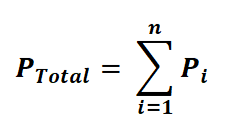
Alternatively, we can express it as:
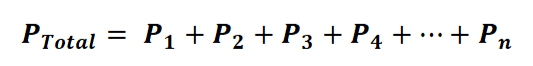
In this equation or formula, p1 + p2 + p3 + p4 + p5 ... + pn represents the partial pressure of each gas in the mixture. Moreover, we can establish a connection between Dalton's Law and the ideal gas law with the following equation:
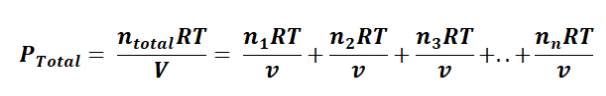
In this context, the common term RTV is consistent for all gases, simplifying the expression to:
nTotal = n1 + n2 + n3 + n4 + n5 + ... + nn
This means that the total number of moles in a gas mixture is the sum of the moles of each individual gas.
Dalton's Law Uses
It finds application in determining gas mixtures, as well as the pressure and volume of individual gases, making it crucial in the chemical industry. Modern industries rely on advanced software for these calculations, but the foundational principles of Dalton's and Avogadro's laws underpin these technologies.
Dalton's Law Formula Example
Let's consider a mixture of nitrogen gas, helium gas, and argon gas where the total pressure is 2 atm. The technician determined that the pressure of nitrogen in the mixture is 0.8 atm, and the pressure of helium is 0.5 atm. Now, we need to find the pressure of argon gas in the mixture.
Solution: To calculate the pressure, we apply Dalton's Law, expressed as:
p total = p 1 + p 2 + p 3 + p 4 + p 5 + ... + p n
Substituting the given values and rearranging the formula, we have:
p total = p nitrogen + p helium + p argon
Now, to find the pressure of argon, we isolate it:
p argon = p total - p nitrogen - p helium
Substituting the values:
pargon = 2 atm - 0.8 atm - 0.5 atm
So, the pressure of argon gas in the mixture is:
pargon = 0.7 atm
Consideration
While Avogadro's law is widely used for industrial purposes, it's important to note that there are optimizations needed in this ideal gas model. The ideal gas concept does not account for factors considered in equations like the Van der Waals equation, which is designed to provide a more accurate description of the real behavior of certain gases.
Dalton's Law Formula Solved Example
Question: In a container of 500 mL, there is a mixture of three gases: argon, nitrogen, and hydrogen. The pressure of nitrogen is 128 torr, hydrogen is 650 torr, and argon is 345 torr. Calculate the total pressure in the container.
Solution: Following Dalton's law, the sum of pressure results from the addition of the partial pressures of each gas:
p total = p nitrogen + p hydrogen + p argon
Substituting the given pressures:
ptotal = 128 torr + 650 torr + 345 torr
Hence, the total pressure in the container is:
ptotal = 1125 torr
Dalton's law, a fundamental principle in gas behavior, helps us understand how gases behave in mixtures. It states that the total pressure in a gas mixture is the sum of the partial pressures of each individual gas component. This law is a valuable tool in various applications, including chemistry, industry, and understanding the behavior of gases in different contexts. By applying Dalton's law, we can effectively calculate and predict the behavior of gas mixtures, making it an essential concept in the study of gases.
| Related Links | |
| Molybdic Acid Formula | Iron (II) Oxide Formula |
| Hydrochloric Acid Formula | Hydrofluoric Acid Formula |
Dalton's Law Formula FAQs
What is Dalton's Law Formula?
What does the Dalton's Law Formula help us calculate?
How can Dalton's Law be applied in real-life situations?
How does Dalton's Law relate to the behavior of gases in mixtures?










