
Hydrofluoric Acid Formula: One of the most significant acid formulas to know is the Hydrofluoric Acid formula. This formula combines hydrogen and fluorine through a single ionic bond.
Hydrogen is a basic chemical element represented by the symbol H. It holds the atomic number 1 and has an electronic configuration of 1s1. It's the lightest element and is colorless, odorless, tasteless, and non-toxic. Hydrogen naturally occurs in molecular form, consisting of one proton and one electron. It can be produced from various common sources like natural gas, biomass, solar energy, and wind.
Fluorine is another chemical element, a non-metal with the symbol F. Its atomic number is 9, and its electronic configuration is 1s2 2s2 2p5. Fluorine appears as a yellowish gas and is the first element in the halogen gas family. It's naturally found in the Earth's crust, present in substances like coal, clay, and rocks. Fluorine has various everyday uses, such as in refrigerators, toothpaste, and rocket fuels. Additionally, it plays a critical role in producing nuclear materials for nuclear power plants.
Hydrofluoric Acid Formula
Hydrofluoric Acid, or HF for short, is a powerful acid. Its formula combines fluorine and hydrogen, making it one of the strongest inorganic acids. This acid is unique because it's a colorless gas that can dissolve in water. It was first discovered by Carl Wilhelm Scheele back in 1771.
HF serves various purposes in different industries. It's used to create herbicides and pharmaceuticals. In the industrial world, it plays a crucial role in tasks like manufacturing electronics, etching glass, and cleaning metal surfaces. Moreover, HF is an essential ingredient in the production of everyday items, including fluorescent light bulbs, electronic components, and plastics.
Hydrofluoric Acid Formula Charge
Hydrofluoric Acid (HF) is a covalent molecule, and it does not possess a net electrical charge or ionic characteristics. Each hydrogen atom contributes one positive charge, and each fluorine atom contributes one negative charge, effectively cancelling each other out, resulting in a neutral molecule. The difference in electronegativity between hydrogen and fluorine results in the formation of a polar covalent bond within the molecule.
Hydrofluoric Acid Formula in Aqueous Solution
When Hydrofluoric Acid (HF) is dissolved in water, it forms a solution of aqueous hydrofluoric acid. In this solution, HF molecules partially ionize, producing hydronium ions (H3O+) and fluoride ions (F-) in the water. This makes the solution acidic due to the presence of H3O+ ions.
Hydrofluoric Acid Formula Structure
The structure of Hydrofluoric Acid is described by the Hydrofluoric Acid Formula is HF. To create its Lewis structure, follow these steps:
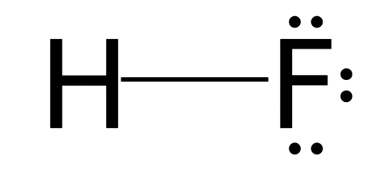
First, determine the total number of valence electrons. Hydrogen, found in the 1st group, has one valence electron, and fluorine has seven valence electrons. So, the total comes to 8 valence electrons for the HF Lewis structure.
Since there's no central atom in this molecule, we'll just write H and F.
Now, place a pair of electrons between the atoms to represent the chemical bond. This bond is called a covalent bond and connects the hydrogen and fluorine atoms.
Hydrofluoric Acid Formula Physical Properties
- Molecular weight: 20.006 g/mol
- Density: 1.15 g/mol
- Melting point: -83.6°C
- Boiling point: 19.5°C
Hydrofluoric Acid Formula Chemical Properties
Chemical formula: HF
Hydrofluoric Acid has various chemical reactions:
It reacts with silicon dioxide to produce silicon tetrafluoride and water:
SiO 2 + 4 HF → 2 H 2 O + SiF 4
It reacts with silicon to create silicon tetrafluoride and water:
Si + 4 HF → 2 H 2 + SiF 4
When it reacts with potassium, it forms potassium fluoride:
2 K + 2 HF → H 2 + 2 KF
Sodium hydroxide reacts with Hydrofluoric Acid to produce sodium fluoride and water:
NaOH + HF → H 2 O + NaF
When it reacts with magnesium, it results in the formation of magnesium fluoride:
Mg + 2 HF → H 2 + MgF 2
Hydrofluoric Acid Preparation
To prepare Hydrofluoric Acid, various chemical reactions can be used:
When fluorine reacts with ammonia, it forms dinitrogen and hydrofluoric acid:
2 NH 3 + 3 F 2 → N 2 + 6 HF
Fluorine can also react in water to produce Hydrofluoric Acid:
2 H 2 O + 2 F 2 → O 2 + 4 HF
Hydrofluoric Acid can be obtained by the reaction of hydrogen with fluorine:
H 2 + F 2 → 2 HF
Another method involves the reaction of fluorine with sulfuric acid, resulting in sulfur hexafluoride and hydrofluoric acid:
H 2 SO 4 + 4 F 2 → 2 O 2 + 2 HF + SF 6
Health Effects of Hydrofluoric Acid
Hydrofluoric Acid can lead to irritation of the eyes, nose, and respiratory tract.
Exposure to it may result in severe electrolyte imbalances.
It can cause intense pain at the point of contact.
Prolonged exposure may lead to lung disease.
Eye exposure can result in permanent visual defects.
Hydrofluoric Acid Safety Measures
When working with Hydrofluoric Acid, always wear protective clothing.
Use gloves that cover not only the hands but also the wrists and forearms.
Wear protective eyeglasses to shield the eyes.
Put on an acid-resistant apron for added protection.
Ensure that all containers of HF are clearly labeled for easy identification.
In case of contact, immediately flush the eyes and hands with water to minimize potential harm.
Uses Hydrofluoric Acid
Hydrofluoric Acid is used in the manufacturing of herbicides and pharmaceuticals.
It serves as an effective cleaning agent in various industrial applications.
It is a crucial ingredient in the production of stain removers.
It functions as a reagent for dissolving silicates and oxides.
Hydrofluoric Acid is utilized in the production of electrical components and fluorescent light bulbs.
| Related Links | |
| Hydrogen Gas Formula | Hydroiodic Acid Formula |
| Nitrogen Dioxide Formula | Hydrobromous Acid Formula |
Hydrofluoric Acid Formula FAQs
What is the chemical formula for Hydrofluoric Acid?
Is Hydrofluoric Acid a strong acid?
What happens when HF is dissolved in water?
What are the common uses of Hydrofluoric Acid?










