
Hydrochloric Acid Formula: Hydrogen (H) has unique properties that set it apart from all other elements found on Earth. Approximately two-thirds of the mass in our Universe consists of this distinctive element. Hydrogen exhibits both electropositive and electronegative qualities, as it forms hydrogen ions (H+) and hydride ions (H–) in its structures. Hydrogen compounds serve as the primary oxidizers for various substances in the atmosphere and play a crucial role in the characteristics of numerous chemical families. Hydrogen is also used in the production of ammonia (NH3).
Chlorine (Cl) is a gas with a greenish-yellow color and a pungent, distinctive odor.This gas boiling point is 239.11K and melting point is 171.6K, it's bad for the environment and is much heavier than regular air, being 2-5 times denser. With an atomic number of 17, chlorine ranks as the second-lightest halogen among all the elements. It possesses two stable isotopes. When subjected to the oxidation process, chlorine can be used for bleaching, requiring moisture for its bleaching action.When chlorine combines with water, it results in the creation of both hydrochloric acid and hypochlorous acid.
Hydrochloric Acid Formula
Hydrochloric acid (HCl) is a strong corrosive acid made of two elements: hydrogen and chlorine. Some people also call it hydrogen chloride or muriatic acid. It's a simple molecule made of only two atoms, one from chlorine and the other from hydrogen. They stick together with a covalent bond, which is a bit polar because the chlorine atom is more electronegative than the hydrogen atom. This acid is incredibly strong and has a distinctive colorless appearance with a sharp, pungent odor.
The Formula of Hydrochloric Acid
Hydrochloric acid consists of two elements: Hydrogen and Chlorine. It's represented by this chemical formula:
H + Cl → HCl
Hydrochloric Acid Formula Name
The chemical name for the compound HCl is "Hydrochloric Acid." It is sometimes referred to as "Muriatic Acid" in its concentrated form.
Hydrochloric Acid Formula Charge
The chemical formula for hydrochloric acid is HCl, consisting of one hydrogen atom (H) and one chlorine atom (Cl). It is a binary compound with a net charge of zero, as the hydrogen atom has a +1 charge, and the chlorine atom has a -1 charge, resulting in a balanced, neutral compound.
Hydrochloric Acid Formula Valency
In the chemical formula for hydrochloric acid (HCl), hydrogen (H) has a valency of +1, and chlorine (Cl) has a valency of -1. This results in a balanced compound, as the positive valency of hydrogen cancels out the negative valency of chlorine, making the overall compound neutral.
Hydrochloric Acid Formula Structure
Hydrogen is connected to the chlorine atom through a covalent bond, and it has a flat, planar structure. The structure of hydrochloric acid, also known as muriatic acid, can be shown as:
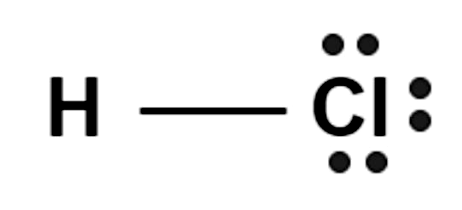
Preparation of Hydrochloric Acid
Hydrochloric acid is produced by heating Sodium Chloride (NaCl) with concentrated sulphuric acid (H 2 SO 4 ). The resulting gas can be dried by passing it through concentrated sulphuric acid. The chemical reactions involved are as follows:
NaCl + H 2 SO 4 → NaHSO 4 + HCl
NaHSO 4 + NaCl → Na 2 SO 4 + HCl
Hydrochloric Acid Formula Physical Properties
Hydrochloric acid exhibits the following characteristics:
Molar Mass: Its molecular weight is 36.458 g/mol.
Physical State: It presents itself as a clear, transparent liquid.
Variable Boiling and Melting Points: The boiling point and melting point of hydrochloric acid differ based on its concentration.
Hydrogen Chloride Component: Hydrogen chloride, a constituent of hydrochloric acid, is a colorless gas with a potent odor.
Reactivity with Metals: Gaseous hydrogen chloride can engage in reactions with active metals and their hydroxides, carbonates, and oxides, leading to the formation of chlorides. In its gaseous state, hydrogen chloride remains non-reactive and completely dry.
Corrosiveness: Similar to hydrogen gas in metal reactions, hydrochloric acid is corrosive in nature.
Effects on Materials: While glass remains unaffected by hydrochloric acid, the acid itself is highly corrosive and can corrode various metals such as platinum, gold, silver, mercury, and tantalum.
Monoprotic Nature: Hydrochloric acid is monoprotic, signifying that it can release only one proton.
Dissociation in Water: When dissolved in water, hydrochloric acid undergoes complete dissociation, resulting in the formation of hydrogen and chloride ions.
Hydrochloric Acid Formula Chemical Properties
Hydrochloric acid can be oxidized with substances like potassium permanganate (KMnO 4 ) or potassium dichromate (K 2 Cr 2 O 7 ), resulting in the formation of chlorine gas.
2KMnO 4 + 16 HCl → 2KCl + 2MnCl 2 + 5Cl 2 + 8H 2 O
2K 2 Cr 2 O 7 + 14 HCl → 2KCl + 2CrCl 3 + 3Cl 2 + 7H 2 O
When hydrochloric acid reacts with salts like carbonates, hydrogen carbonates, and sulfites, it produces carbon dioxide gas and sulfur dioxide gas, respectively.
Na 2 CO 3 + 2 HCl → 2NaCl + H2O + CO 2
NaHCO 3 + HCl → NaCl + H 2 O + CO 2
Na 2 SO 3 + 2 HCl → 2NaCl + H 2 O + SO 2
A mixer concentrates HCl and concentrates HNO 3 in a 3:1 volume ratio, it's known as Aqua regia. When HCl dissolves metals like gold and platinum, they form soluble chlorides.
Uses of Hydrochloric Acid
Purification of Table Salt and pH Control: Hydrochloric acid is used to purify table salt. It is also valuable in controlling the acidity of solutions and is used for regulating the pH of pharmaceutical products, drugs, water, and food items.
Oil Production: Hydrochloric acid is injected into rock formations, creating large-pore structures that enhance oil production.
Cleaning Agent: Thanks to its corrosive properties, hydrochloric acid is an effective stain and rust remover for metals like copper and iron. It is used for cleaning tiles in bathrooms and kitchens and also serves as a disinfectant.
Inorganic Compounds: Hydrochloric acid is used in the preparation of inorganic compounds that serve as water treatment chemicals. For example, it is used in the production of substances like polyaluminium chloride (PAC), ferric chloride, and aluminum-based chemicals for water treatment. Additionally, it is used in the regeneration of ion-exchange resins.
Gastric Acid: Hydrochloric acid is a crucial component of gastric juice in the human body, aiding in digestion. It converts inactive pepsinogen into active pepsin in the stomach, which facilitates the digestion process by breaking down the bonds between amino acids. This process is known as Proteolysis.
Pickling of Steel: Diluted hydrochloric acid is used in the pickling process to remove rust and iron oxide from steel or iron before it is processed into wire, sheet and strip coatings, and tin-plated products.
Organic Compounds: Hydrochloric acid plays a role in producing organic compounds such as vinyl chloride and dichloromethane, which are essential for manufacturing PVC (polyvinyl chloride). It's also used in making things like ascorbic acid and different medicines.
| Related Links | |
| Molybdic Acid Formula | Iron (II) Oxide Formula |
| Iron (II) Sulfate Formula | Iron (III) Oxide Formula |
Hydrochloric Acid Formula FAQs
. What is the chemical formula for hydrochloric acid?
How is hydrochloric acid prepared?
What are the physical properties of hydrochloric acid?
What are the chemical properties of hydrochloric acid?
What is the primary use of hydrochloric acid in the human body?










