Electronic Configurations: The electron configuration represents the arrangement of electrons within the atomic or molecular orbitals of an atom, molecule, or a similar structure. For instance, the electron configuration of a neon atom is written as 1s² 2s² 2p⁶, indicating that the 1s, 2s, and 2p subshells hold 2, 2, and 6 electrons, respectively.
Electronic Configurations of Neon: 1s² 2s² 2p
In electronic configurations, we imagine that each electron moves on its own in an orbital, influenced by the combined pull of all the other nearby orbits.
Electronic Configurations
The standard notation can generate extended electron configurations, particularly for elements with higher atomic numbers. To reduce this, a concise or condensed form may be used instead of the standard notation. This condensed approach replaces the sequence of completely filled subshells, akin to the electronic configuration of a noble gas, with the noble gas symbol enclosed in square brackets. Consequently, sodium's concise electron configuration becomes [Ne]3s¹ (noting that neon's configuration is 1s²2s²2p⁶, abbreviated as [He]2s²2p⁶). Below are some electronic Configuration.

Electron Configurations serve the following purposes:
Assessing the valency of an element.
Determine the characteristics of groups of elements (as elements with akin electron configurations often display similar properties).
Interpreting atomic spectra.
This method of representing the distribution of electrons in atomic orbitals emerged shortly after the introduction of the Bohr model of the atom by Ernest Rutherford and Niels Bohr in 1913.
Expressing Electron Configurations
Below we are describing the Shells, Subshell and Notation of Electronic Configuration.
Shells
The capacity of electrons within a shell is determined by the principal quantum number (n), indicated by the formula 2n², where 'n' denotes the Shell number. The shells, their corresponding 'n' values, and the maximum count of electrons they can house are presented in the table below.
| Shell and ‘n’ value | Maximum electrons present in the shell |
| K shell, n=1 | 2*12 = 2 |
| L shell, n=2 | 2*22 = 8 |
| M shell, n=3 | 2*32 = 18 |
| N shell, n=4 | 2*42 = 32 |
Subshells
The distribution of electrons into subshells is determined by the azimuthal quantum number (represented by 'l').
This quantum number depends on the principal quantum number, n. Hence, when n equals 4, four distinct subshells are possible.
For n=4, the subshells are characterized by l=0, l=1, l=2, and l=3, denoted as the s, p, d, and f subshells, respectively.
The maximum electron capacity of a subshell is calculated by the formula 2*(2l + 1).
Hence, the s, p, d, and f subshells can house a maximum of 2, 6, 10, and 14 electrons, respectively.
A comprehensive table featuring all potential subshells for values of n up to 4 is outlined below.
| Principle Quantum Number Value | Value of Azimuthal Quantum Number | Resulting Subshell in the Electron Configuration |
| n=1 | l=0 | 1s |
| n=2 | l=0 | 2s |
| l=1 | 2p | |
| n=3 | l=0 | 3s |
| l=1 | 3p | |
| l=2 | 3d | |
| n=4 | l=0 | 4s |
| l=1 | 4p | |
| l=2 | 4d | |
| l=3 | 4f |
Hence, it is apparent that orbitals such as 1p, 2d, and 3f do not exist due to the consistent relationship where the value of the azimuthal quantum number is always less than that of the principal quantum number.
Notation Approach
The electron configuration of an atom is written using subshell labels.
These labels encompass the shell number (derived from the principal quantum number), the subshell Name (derived from the azimuthal quantum number), and the total count of electrons in superscript within the subshell.
For instance, if two electrons occupy the 's' subshell of the initial shell, it is represented as '1s²'.
Utilizing these subshell labels, the electron configuration of magnesium (with an atomic number of 12) can be expressed as 1s² 2s² 2p⁶ 3s².
Filling Atomic Orbitals
Below we are describing the Aufbau principle, Pauli Exclusion Principle and Hund’s Rule of Electronic Configuration.
The Aufbau Principle
Named after the German term 'Aufbeen', signifying 'build-up,' the Aufbau principle derived the sequence in which electrons populate orbitals within an atom.
This principle stipulates that electrons occupy orbitals of lower energy levels before filling those of higher energy levels. An orbital's energy is determined by the sum of the principal and azimuthal quantum numbers.
Accordingly, electrons are arranged in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p…
The sequence detailing the order in which electrons fill atomic orbitals, in accordance with the Aufbau principle, is outlined below.
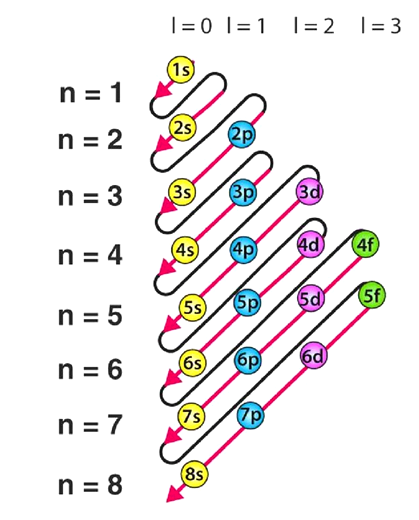
It's crucial to recognize numerous exceptions to the Aufbau principle, like those observed in chromium and copper. These exceptions find the explanation in the stability gained from half-filled or completely filled subshells.
Pauli Exclusion Principle
The Pauli exclusion principle outlines that a maximum of two electrons, each with opposite spins, can occupy a single orbital within an atom. This principle can be summarily expressed as "no two electrons within the same atom can share identical values for all four quantum numbers."
Hence, if the principal, azimuthal, and magnetic quantum numbers are identical for two electrons, they necessitate having opposite spins as mandated by this principle.
Hund’s Rule
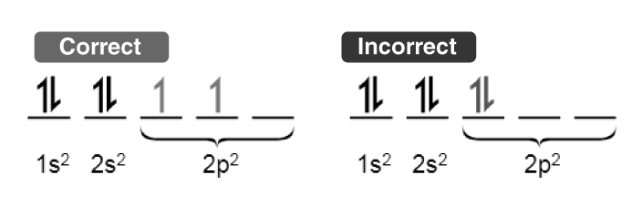
This rule defines the sequence in which electrons populate the orbitals within a particular subshell. It states that each orbital in the subshell is first singly occupied by electrons before a second electron is added to any of them.
To maximize the total spin, electrons within orbitals containing only one electron all adopt the same spin (or share identical values of the spin quantum number).
Illustrated above is a demonstration of the adherence to Hund’s rule for maximum multiplicity when filling electrons.
Representation of Atomic Electron Configurations
Within this section, illustrations demonstrating the electron configurations of select elements are presented.
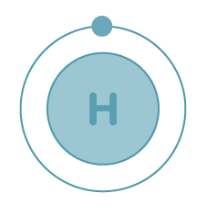
Hydrogen, with an atomic number of 1, holds one electron. This electron is situated in the subshell of the initial shell/orbit.
Electronic Configuration: The electron configuration of hydrogen is represented as 1s¹, as displayed below.
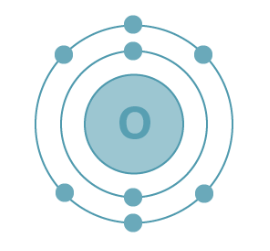
Electron Configuration of Oxygen
Oxygen holds an atomic number of 8, indicating the presence of 8 electrons. The filling order for its electrons is as follows:
K shell – 2 electrons
L shell – 6 electrons
Electronic Configuration of Oxygen: The electron configuration of oxygen is depicted as 1s² 2s² 2p⁴, as illustrated below.
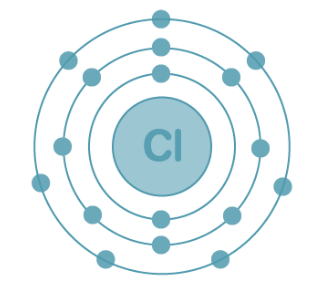
Electronic Configuration of Chlorine
With an atomic number of 17, chlorine accommodates 17 electrons arranged as follows:
K shell – 2 electrons
L shell – 8 electrons
M shell – 7 electrons
Electronic Configuration of Chlorine: The electron configuration of chlorine is presented below and can be represented as 1s²2s²2p⁶3s²3p⁵ or in the abbreviated form [Ne]3s²3p⁵.
Join Online Course of Class 9 Neev Fastrack 2024 and Class 10 Udaan Fastrack 2024 to enhance your chemistry knowledge. and build a strong foundation.
| Related Links | |
| Carbon Disulfide Formula | Sodium Thiosulfate Formula |
| Silver Phosphate Formula | Chloroauric Acid Formula |
Electronic Configuration FAQs
What is an electron configuration?
Why are electron configurations important?
How is an electron configuration written?
What is the significance of the "s," "p," "d," and "f" orbitals in electron configurations?