
Silver Phosphate Formula: Silver Phosphate, also referred to as Silver Orthophosphate and with its IUPAC name as Silver(1)Phosphate, possesses a cubic crystal structure. This inorganic salt, denoted as Ag 3 PO 4 , comprises three silver cations (Ag + ) and one phosphate anion. It is important to note that Silver Phosphate is not naturally occurring in the environment. It is synthesized within a laboratory setting through a reaction between Silver Nitrate and Orthophosphate salt. This compound takes the form of a yellow solid that is insoluble in water and is recognized for its antibacterial properties.
Furthermore, Silver Phosphate finds application as a therapeutic agent in the treatment of cancer patients. However, when subjected to combustion, Silver Phosphate emits toxic fumes, leading to eye irritation, respiratory ailments, skin irritation, and coughing.
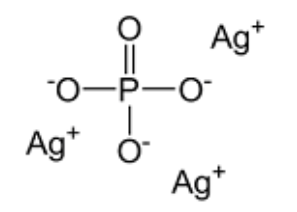
Silver Phosphate Formula Structure
The structure of Silver(I) Phosphate is depicted below, consisting of three Silver Cations and one Phosphate Anion.
Silver Phosphate Formula Physical Properties
Color: Silver Phosphate presents as a solid with a yellow hue.
Odor: This compound is devoid of any discernible odor.
Molar Mass: The molar mass of Silver Phosphate is 419 g/mol.
Solubility: Silver Phosphate is insoluble in water but shows moderate solubility in dilute acetic acid. It is also soluble in dilute HNO 3 , ammonia, thiosulphates, and ammonium carbonate.
Flammability: It is non-flammable and does not produce soot.
Density: The density of Silver Phosphate is 6.38 g/mL.
Melting Point: It exhibits a melting point of 848 degrees Celsius.
Silver Phosphate Formula Chemical Properties
Formation of Silver Pyrophosphate:
When silver nitrate is combined with phosphate ions at elevated temperatures, a condensation reaction occurs, resulting in the creation of silver pyrophosphate.
Reaction with Potassium Permanganate:
Silver Phosphate, when reacted with potassium permanganate, undergoes a double displacement and redox reaction, yielding silver permanganate and tribasic potassium phosphate as products.
Ag 3 PO 4 + 3KMnO 4 → 3AgMnO 4 + K 3 PO 4
Reaction with Nitric Acid:
When Silver Phosphate interacts with nitric acid, it transforms into silver nitrate and phosphoric acid.
HNO 3 + Ag 3 PO 4 → H 3 PO 4 + AgNO 3
Reaction with Ammonium Hydroxide:
Silver Phosphate, when reacted with ammonium hydroxide, forms diamine silver phosphate and water in the reaction.
Ag 3 PO 4 + 6(NH 3 ·H 2 O) → [Ag(NH 3 ) 2 ]3PO 4 + 6H 2 O
These chemical reactions illustrate the versatile behavior of Silver Phosphate when exposed to various reagents, resulting in the formation of different compounds with distinct properties.
Preparation of Silver Phosphate
Silver Phosphate can be obtained through various methods, primarily involving the reaction of silver nitrate with different phosphate-containing compounds. The resulting product is Silver Phosphate, characterized by its insolubility in water and its purity as a yellow solid.
Reaction with Sodium Orthophosphate:
When silver nitrate (AgNO 3 ) reacts with sodium orthophosphate (Na 3 PO 4 ), Silver Phosphate is formed, accompanied by the production of sodium nitrate (NaNO 3 ).
AgNO 3 + Na 3 PO 4 → Ag 3 PO 4 (S) + 3NaNO 3
Reaction with Potassium Orthophosphate:
The synthesis of Silver Phosphate can also be achieved by reacting silver nitrate (AgNO3) with tripotassium phosphate (K 3 PO 4 ), leading to the formation of Silver Phosphate and potassium nitrate (KNO 3 ).
K 3 PO 4 + 3AgNO 3 → Ag 3 PO 4 + 3KNO 3
Reaction with Phosphoric Acid:
Another method for the preparation of Silver Phosphate involves the reaction between silver nitrate (AgNO 3 ) and phosphoric acid (H 3 PO 4 ), resulting in the formation of Silver Phosphate and nitric acid (HNO 3 ).
H 3 PO 4 + 3AgNO 3 → Ag 3 PO 4 + 3HNO 3
Reaction with Lead Phosphate:
Silver Phosphate can also be synthesized by reacting silver nitrate (AgNO 3 ) with lead phosphate (Pb 3 (PO 4 ) 2 ), yielding Silver Phosphate and lead nitrate (Pb(NO 3 ) 2 ).
Pb 3 (PO 4 ) 2 + AgNO 3 → Ag 3 PO 4 + Pb(NO 3 ) 2
These reactions are efficient methods for producing Silver Phosphate, which can then be collected as a pure yellow solid by filtration.
Harmful Effects of Silver Phosphate
Inhalation Hazards: When burned, Silver Phosphate emits toxic fumes that can lead to eye irritation, respiratory ailments, and coughing.
Skin Irritation: Direct contact with Silver Phosphate can cause skin irritation.
It is essential to handle Silver Phosphate with care and take necessary precautions to mitigate these adverse effects, especially in situations where it may be subjected to high temperatures or close human contact.
Uses of Silver Phosphate
Antibacterial Agent: Silver Phosphate is used in various materials due to its antibacterial properties, making it valuable for applications requiring antimicrobial properties.
Catalyst: It serves as a catalyst in chemical reactions, facilitating and accelerating these processes.
Anti-Inflammatory Agent: It is used as an anti-inflammatory substance, contributing to its medical applications.
Anticancer Activity: Silver Phosphate exhibits anticancer properties and is harnessed as a therapeutic drug in the treatment of cancer.
Silver Staining: It is used for silver staining of biological materials, aiding in the visualization and analysis of various biological samples.
Medical and Pesticidal Uses: Silver Phosphate finds utility in the medical field and is used in the formulation of pesticides.
Photography: In the realm of photography, it is used as an emulsion because of its light sensitivity, contributing to photographic processes.
Phosphate Concentration Determination: Silver Phosphate is utilized in analytical chemistry to assess the concentration of phosphate ions in solutions.
| Related Links | |
| Potassium Nitrate Formula | Potassium Iodate Formula |
| Potassium Hexacyanoferrate (III) Formula | Fluorine Gas Formula |
Silver Phosphate Formula FAQs
What is the chemical formula for Silver Phosphate?
What is the crystal structure of Silver Phosphate?
Is Silver Phosphate naturally occurring?
What is the primary method for preparing Silver Phosphate?
What is the color of Silver Phosphate?










