
Carbon Disulfide Formula: Carbon disulfide, denoted by the chemical formula CS₂ and the structure S=C=S, is an inorganic compound. This colorless, flammable liquid, characterized as neurotoxic, serves as a fundamental component in organic synthesis.
Carbon Disulfide was initially uncovered by the German chemist Wilhelm August Lampadius. In 1796, Lampadius was the first to synthesize this compound by heating pyrite with moist charcoal. He referred to it as "liquid sulfur." The definitive composition of Carbon Disulfide was established in 1813 by a collaborative effort involving the Swedish chemist Jons Jacob Berzelius and the Swiss-British chemist Alexander Marcet.
Carbon Disulfide Formula
It's also known as Bisulfide, and Carbon Disulfide Formula is CS 2 . This substance is a colorless, toxic, highly volatile, and flammable liquid compound that is poisonous.
Carbon Disulfide is widely used in organic chemistry as a fundamental building block, as well as a non-polar industrial and chemical solvent. It is also naturally generated through anaerobic biodegradation and can be emitted into the atmosphere from landmasses, oceans, and geothermal sources. Coastal and marshland regions with high biological activity, along with the ocean, are recognized as significant sources of carbon disulfide.
What is Carbon Disulfide
This compound results from the combination of carbon and sulfur, comprising one mole of carbon and two moles of sulfur. Carbon Disulfide forms when coke reacts with sulfur at elevated temperatures. It is a hazardous substance with detrimental effects on the human body, affecting the skin, liver, eyes, cardiovascular system, and kidneys.
Additionally, it emits an offensive odor and is a neurotoxic, colorless, volatile liquid. Its production and use as a chemical intermediate and solvent may lead to its release into the environment through various waste streams. Furthermore, its use as an insecticide can result in direct environmental release.
Carbon Disulfide Formula Structure
The structural formula of Carbon Disulfide is as follows:
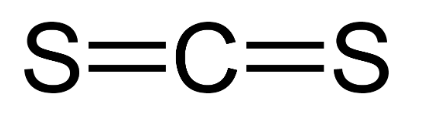
Carbon Disulfide Formula Physical Properties
- It possesses a molar mass or molecular weight of 76.13 g/mol.
- it has a boiling point of 46.24°C.
- The melting point is -111.61°C.
- Its density measures 1.539 g/cm3.
Carbon Disulfide Formula Chemical Properties
Carbon disulfide is insoluble in water and is denser than water.
It readily evaporates at room temperature, and its vapor is over twice as heavy as air.
When it reacts with oxygen, it produces carbon dioxide and sulfur dioxide:
CS 2 + 3O 2 → CO 2 + SO 2
In the presence of sodium sulfide, it forms trithiocarbonate:
Na 2 S + CS 2 → [Na + ] 2 [CS 3 2- ]
Reduction with sodium yields sodium 1,3-dithiole-2-thione-4,5-dithiolate, along with sodium trithiocarbonate:
4Na + 4CS 2 → Na 2 C 3 S 5 + Na 2 CS 3
Chlorination of carbon disulfide provides a pathway to carbon tetrachloride:
CS 2 + 3Cl 2 → CCl 4 + S 2 Cl 2
Carbon Disulfide Formula Preparation
The main source of carbon disulfide in the environment is attributed to rayon factories, with a significant portion of carbon disulfide emissions originating from rayon production. Other notable sources include the manufacturing of cellphones, carbon tetrachloride, carbon black, and sulfur recovery processes.
CS 2 is produced by the high-temperature combination of carbon and sulfur, described as follows:
C + 2S →CS 2
Alternatively, at lower temperatures, natural gas (CH 4 ) serves as the carbon source in the presence of a silica gel or alumina catalyst:
2CH 4 + S → 2CS 2 + 4H 2 S
Uses of Carbon Disulfide Formula
Carbon disulfide finds utility in numerous manufacturing sectors and processes. Its versatile applications include:
Rayon Production: Carbon disulfide plays a crucial role in the manufacture of rayon fibers, a synthetic material used in textiles.
Solvent for Iodine and Phosphorus: It serves as a solvent in the handling and processing of iodine and phosphorus, contributing to various chemical processes.
Electronic Vacuum Tubes: In the production of electronic vacuum tubes, carbon disulfide is used as an integral component for specific applications within the electronics industry.
Insecticide for Grain Fumigation: Carbon disulfide is used as an insecticide for the fumigation of grains, protecting stored crops from pests and infestations.
Organosulfur Compound Synthesis: It is widely used in the synthesis of organosulfur compounds, which have diverse applications in the chemical industry.
Carbon Tetrachloride Synthesis: This compound is used in the chemical synthesis of carbon tetrachloride, a chemical with various industrial applications.
Solvent in Rubber Manufacturing: Carbon disulfide is utilized as a solvent in the rubber manufacturing process, aiding in the production of rubber products.
These applications demonstrate the diverse and important roles that carbon disulfide plays in various industrial and manufacturing processes.
| Related Links | |
| Pyrophosphoric Acid Formula | Potassium Sulfite Formula |
| Dinitrogen Pentoxide Formula | Potassium Nitrate Formula |
Carbon Disulfide Formula FAQs
What is the chemical formula for Carbon Disulfide?
Who first discovered Carbon Disulfide?
What are the key properties of Carbon Disulfide?
Is Carbon Disulfide soluble in water?
What happens when Carbon Disulfide reacts with oxygen?










