
Sodium Thiosulfate Formula is Na 2 S 2 O 3 . Sodium, represented by the symbol Na, is an extremely reactive metallic element derived from the Latin word "natrium." It possesses a characteristic soft, silvery-white appearance and is identified by its atomic number, 11. Sodium belongs to Group 1 of the periodic table. It is classified as an alkali metal, with an atomic mass of approximately 22.989 unified atomic mass units (u) and a mass number of 23 atomic mass units (amu).
Oxygen, an essential member of the chalcogen group in the periodic table, is a highly reactive non-metal. It manifests as a colorless, odorless gas crucial for the survival of living organisms. Its atomic weight is approximately 15.999 u, and it is recognized by the chemical symbol O with an atomic number of 8.
Sulfate, sometimes spelled as sulphate, represents an ion that is a salt originating from sulfuric acid, with a molar mass of roughly 96.06 grams per mole (g/mol). It is soluble in water and is a divalent anion formed through the oxidation of elemental sulfur and organic sulfur compounds. The empirical formula for sulfate is SO 4 2- .
Sodium Thiosulfate Formula
Sodium Thiosulfate, also known as sodium hyposulfite, is a crystalline compound containing five water molecules. It holds significance due to its remarkable solubility, transparent and odorless nature. This inorganic salt is also referred to as disodium thiosulfate and is represented by the chemical formula Na 2 S 2 O 3 , possessing a molar mass of approximately 158.11 g/mol. It finds numerous applications and exhibits various medical properties.
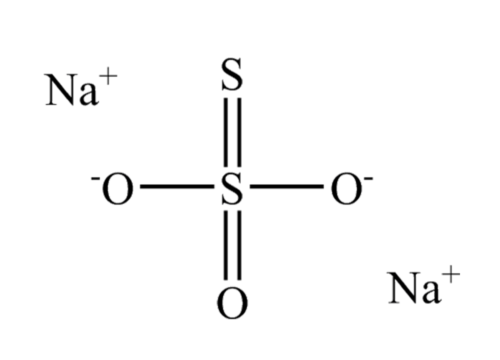
The chemical formula for sodium thiosulfate, Na 2 S 2 O 3 , is comprised of two sodium cations (Na + ) and an anion of thiosulfate (S 2 O 3 – ), which involves sulfur (S) and oxygen (O) atoms.
Sodium Thiosulfate Common Name
Sodium thiosulfate is commonly referred to as "hypo," derived from its historical name, the "hyposulfite of soda." This inorganic salt holds significant medical applications and is also known by the name "sodium hyposulfite" or simply "hypo."
Sodium Thiosulfate Formula Structure
Within the structure of sodium thiosulfate, the central sulfur atom forms bonds with three oxygen atoms and another sulfur atom. In its solid state, it adopts a monoclinic crystal structure.
Preparation of Sodium Thiosulfate
The typical method for preparing sodium thiosulfate involves heating sulfur in the presence of an aqueous solution of sodium sulfite. The reaction can be represented as follows:
6NaOH + 4S → Na 2 S 2 O 3 + 2Na 2 S + 3H 2 O
Sodium Thiosulfate Formula Physical Properties
- Sodium thiosulfate is a white crystalline solid in appearance.
- Its melting point is approximately 48.3°C.
- It exhibits high solubility in water.
- Sodium thiosulfate is colorless and odourless in nature.
- The density of sodium thiosulfate is approximately 1.67 gm/cm3.
Sodium Thiosulfate Formula Chemical Properties
Under standard conditions, sodium thiosulfate remains stable. However, when subjected to heat, it undergoes decomposition to yield sodium sulfate and sodium polysulfide as follows:
4Na 2 S 2 O 3 → 3Na 2 SO 4 + Na 2 S 5
In clock reactions or when treated with dilute acids, sodium thiosulfate breaks down to produce sulfur and sulfur dioxide:
Na 2 S 2 O 3 + 2 HCl → 2 NaCl + S + SO 2 + H 2 O
Being a neutral salt, it readily dissociates in water, generating sodium and thiosulfate ions:
Na 2 S 2 O 3 + H 2 O → Na+ + S 2 O 3 –
Uses of Sodium Thiosulfate
- Sodium thiosulfate finds diverse uses in various fields:
- It serves as an antidote for cyanide poisoning.
- It plays an important role in photographic film processing.
- It is used in the extraction of gold.
- It features in the formulation of numerous pharmaceutical preparations.
- Sodium thiosulfate is also utilized in the process of leather tanning.
- It functions as a cleansing agent.
| Related Links | |
| Gold Formula | Phosphate Formula |
| Helium Formula | Hydrazine Formula |
Sodium Thiosulfate Formula FAQs
What is the formula for sodium thiosulfate?
What is the common name for sodium thiosulfate?
What is the molar mass of sodium thiosulfate?
How is sodium thiosulfate prepared?
What is the appearance of sodium thiosulfate in its solid state?










