
Chloroauric Acid Formula: Chloroauric Acid Formula is HAuCl 4 . Chloroauric acid, an orange-yellow solid comprising inorganic compounds, goes by the aliases "gold chloride" and "gold chloride tetrahydrate." It exhibits solubility in water, ethanol, ether, and chloroform. Anhydrous chloroauric acid can be obtained through crystallization using an ethanol solution.
Upon exposure to heat, it decomposes, with decomposition into gold trichloride occurring at 120°C. The addition of a cold potassium hydroxide solution results in the precipitation of gold hydroxide. Furthermore, it can be converted into gold powder by using sulfur dioxide. This compound serves as a precursor to various gold compounds and plays a crucial role in gold purification processes. Due to its corrosive and hazardous nature, caution should be exercised when handling this acid.
Chloroauric Acid Formula for Gold
The chemical formula for chloroauric acid, which contains gold, is HAuCl 4 .Chloroauric Acid Formula
Chloroauric Acid formula is HAuCl 4 , a solid with an orange-yellow, consists of inorganic compounds. Its role extends beyond its chemical composition, as it serves as a starting material for various gold compounds. Additionally, it plays a important role in the process of refining gold. This substance is commonly referred to as gold chloride and gold chloride tetrahydrate. Caution is necessary when handling this acid due to its caustic and potentially harmful nature.
Chloroauric Acid Formula Structure
AuCl 3 is both in its solid and vapor forms, it exists as a dimer. This dimerization is also observed in the bromide compound AuBr3. Each gold (Au) center within the molecule exhibits a square planar geometry. This structural arrangement closely resembles the bitetrahedral structures found in compounds such as AlCl 3 and FeCl 3 . Notably, the majority of the bonding in AuCl 3 is covalent, which is attributed to gold's high oxidation state and its relatively strong electronegativity for a metal.
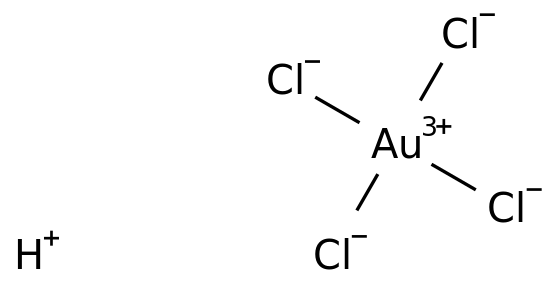
Chloroauric Acid Formula Structure Physical Properties
- IUPAC Name: Hydrontetrachlorogold(1-)
- Molecular Formula: HAuCl 4
- Molecular Mass:
- 339.785 g/mol (anhydrous)
- 393.833 g/mol (trihydrate)
- 411.85 g/mol (tetrahydrate)
- Density:
- 3.9 g/cm3
- 2.89 g/cm3
- Melting Point: 254°C
- Appearance: Yellow-orange needle-like crystals
Chloroauric Acid Formula Chemical Properties
Chloroauric acid is characterized by a high molecular weight and behaves as a monoprotic acid. The crystalline tetrahydrate consists of H 5 O 2 + ions, AuCl 4 ions, and two water molecules. In its solid form, chloroauric acid exhibits hydrophilic and ionic properties, making it a protic solute. It demonstrates strong solubility in water and is also soluble in various oxygen-containing solvents like ethers, esters, alcohols, and ketones. When chloroauric acid is subjected to treatment with a base, it undergoes transformation into water, metal, and tetrachloroaurate. Similarly, the thallium salt exhibits poor solubility in non-reactive solvents, and it is noteworthy that quaternary ammonium cation salts are known to experts. Gold nanostructures can be synthesized from chloroauric acid through a two-phase redox reaction. This reaction involves the simultaneous attachment of self-assembling thiol monolayers on growing nuclei, resulting in the formation of metallic clusters. Partial reduction of chloroauric acid leads to the production of oxonium dichloroisocyanurate(1). Furthermore, in the presence of organic ligands, reduction can give rise to additional gold(I) complexes.Uses of Chloroauric Acid
Gold Purification: Hydrogen tetrachloroaurate(III) serves as a crucial precursor for the electrolytic purification of gold. It plays a important role in processes involving gold recovery, concentration, purification, and analytical determination.
Gold Refinement: Chloroauric acid has traditionally been used in the refinement of gold.
Photographic Gold Toning: Chloroauric acid finds widespread use as a gold toner in photography. Toning is a chemical process that alters the color of photographic images.
Cranberry Glass Production: In the creation of Cranberry glass, often referred to as "Gold Ruby," chloroauric acid or colloidal gold is added to molten glass. This results in the distinctive red color of the glass, which has aesthetic and artistic applications.
Nanoparticle Synthesis: Thanks to advancements in nanotechnology, chloroauric acid is utilized to produce gold nanoparticles with diameters spanning from 5 nm to 400 nm. These nanoparticles find applications in various fields.
| Related Links | |
| Fluorine Gas Formula | Potassium Hexacyanoferrate (III) Formula |
| Potassium Fluoride Formula | Potassium Chlorate Formula |
Chloroauric Acid Formula FAQs
What is the formula for chloroauric acid?
What is the appearance of chloroauric acid?
What is the role of chloroauric acid in gold purification?
In what applications are gold nanoparticles synthesized using chloroauric acid used?
How is chloroauric acid used in photography?










