
Sodium propionate is used to prepare Ethane , a saturated hydrocarbon found in a gaseous state. It contains 2 carbon atoms and 6 hydrogen atoms, making it the second simplest alkane after methane. It is the most important gaseous fuel. Under moderate pressure, natural gas components such as ethane and heavier hydrocarbons can easily be separated from the gas stream and liquefied.
Properties of Ethane
| C 2 H 6 | Ethane |
| Density | 1.36 kg/m³ |
| Molecular Weight/ Molar Mass | 30.07 g/mol |
| Boiling Point | -89 °C |
| Melting Point | -182.8 °C |
| Chemical Formula | C 2 H 6 |
What is Ethane?
- A simple alkane, ethane, has the chemical formula C 2 H 6 .
- This saturated hydrocarbon is typically found in a gaseous state.
- What makes ethane especially useful is its reactivity, easily undergoes various reactions.
- One such reaction is with hydrogen, resulting in the formation of ethylene, the simplest alkene.
- While it is a gas at room temperature, ethane can turn into a liquid when cooled below -78.5 °C.
- This compound occurs naturally through processes like pyrolysis of biomass and bio-loading in marine biology and biogas formation in anaerobic digesters.
- Additionally, it can be produced industrially by steam cracking of ethane, ethylene, or gasoline fractions from petroleum refining.
History of Ethane
The chemist Dmitri Mendeleev, who worked in the 1830s, is credited with discovering ethane. While studying the periodic table, he noticed that an element was missing and should have a formal oxidation state of -2. This observation was later confirmed in 1859 by Mendeleev himself. It wasn't until 1862 that the melting point of ethane, which is 62 °C (147.2 °F), was measured for the first time. In the 19th century, this compound was known as "ether". Although there were some previous findings of ethane, it wasn't until 1897 that Karl Robert Bunsen reported its discovery in its pure form.
Also read : Chemistry Formulas
Synthesis of Ethane – C 2 H 6
The chemical equation for the synthesis of ethane is given below. Ethane is synthesized by reducing ethyl iodide with zinc and copper in alcohol.
CH 3 CH 2 I + 2[H] → C 2 H 6 + HI
Ethane is also formed when methyl bromide or methyl iodide and sodium are heated in dry ether.
CH 3 I + 2Na + CH 3 I → CH 3 – CH 3 + 2NaI
Ethane Structure – C 2 H 6
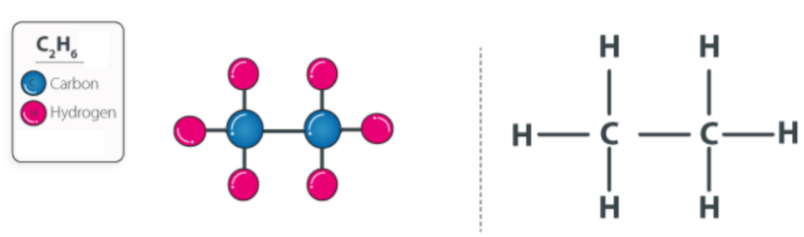
- An ethyl group CH 3 connects two carbon atoms in ethane.
- Due to their two pairs of valence electrons, carbon atoms are highly reactive.
- Two electrons are spare in the ethyl group, which has one pair of valence electrons.
- Carbon has the highest electronegativity among the four elements, meaning it shares electrons with its neighbors most readily.
- Because of this reactivity, the ethyl group can form bonds with carbon atoms.
Also Check – Chemical Bonding Formula
Physical Properties of Ethane
- Gases such as ethane are odorless and colorless.
- Under normal temperature and pressure, it remains colorless, odorless, and highly flammable.
- Natural gas contains 5 - 10% of it.
- Water partially dissolves it, and it is lighter than air.
- The melting point of ethane is -182.8°C, boiling point is 89°C.
- Ethane has a density of 1.05 grams per cubic centimeter.
Also Check – Aluminium Acetate Formula
Chemical Properties of Ethane
- 1 molecule of C 2 H 6 or the molar mass of C 2 H 6 is 30.07 g.
- Ethane acts as raw material in the production of ethylene through the process of pyrolysis: CH 3 CH 3 ⟶ CH 2 CH 2 + H 2
- It can be produced using sodium propionate in the laboratory method.
- The reaction is: CH 3 CH 2 COONa + NaOH → C 2 H 6 + Na 2 CO 3
- Ethane reacts with nitric acid vapor at 400°C to form nitroethane. The reaction for the same is: C 2 H 6 + HONO 2 → C 2 H 5 NO 2 + H 2 O
- Ethane reacts with chlorine in diffused daylight when all the 6 “H” atoms are replaced in turn by chlorine atoms yielding a mixture of various chloro- derivatives.
Uses of Ethane
Here are a few examples of how ethane is used in various industries:
- Plastics, fruit ripening, and detergents are made with ethylene produced from ethane.
- It is the most specific volatile marker available for the investigation of lipid peroxidation.
- Paints, varnishes, adhesives, plastic, and varnishes are made from ethanol, acetaldehyde, and acetic acid.
- Ethane can also be used as a fuel for automobiles when liquified.
Ethane Formula FAQs
Q1. What is the chemical formula for ethane?
Q2. What is the structural formula of ethane?
Q3. Is ethane a natural gas component?
Q4. What are the physical properties of ethane?
Q5. How does ethane differ from methane?










