
Hydrazine Formula: Nitrogen, symbolized as N and found on the periodic table in the fifteenth group, is a common element in nature. It's a gas that makes up a significant portion of Earth's atmosphere. This gas is colorless, odorless, and quite abundant. To produce nitrogen for various industrial uses, a common method is to separate it from liquefied air through a process called fractional distillation. Nitrogen plays a important role in making fertilizers, dyes, explosives, preserving food, and various other industrial applications.
Hydrazine Formula
Hydrazine, chemically represented as N 2 H 2 , goes by several names, such as Diazane, Diamine, and Nitrogen Hydride. It's safe when in the form of Hydrazine hydrate (N 2 H 4 . x H 2 O) , which is a clear, colorless liquid with an ammonia-like smell. However, it's important to handle it with great care since it's highly reactive.Hydrazine Formula Charge
The chemical formula for hydrazine is N 2 H 4 . Hydrazine is a neutral molecule, so it does not have a formal charge. It consists of two nitrogen atoms (N) and four hydrogen atoms (H), and the overall charge is balanced, resulting in a neutral molecule.Hydrazine Formula Decomposition
Hydrazine (N 2 H 4 ) can decompose through various reactions. One common decomposition pathway for hydrazine is as follows:
2N 2 H 4 → N 2 + 3H 2
In this decomposition reaction, hydrazine (N 2 H 4 ) breaks down into nitrogen gas (N 2 ) and hydrogen gas (H 2 ). This reaction is exothermic, meaning it releases heat as it proceeds. The decomposition of hydrazine is often used in rocket propulsion systems as it generates high-temperature gases that can be expelled to create thrust.
Hydrazine Formula Ratio
The chemical formula for hydrazine is N 2 H 4 , which means there is a 2:4 (or simplified as 1:2) ratio of nitrogen (N) atoms to hydrogen (H) atoms in the molecule.Hydrazine Formula Structure
The structure of hydrazine. Hydrazine is represented by the chemical formula N 2 H 4 . In this molecule, each nitrogen atom has 3 valence electrons. Two of these electrons combine with hydrogen atoms, while the remaining one is used to form a single covalent bond between the two nitrogen atoms. In hydrazine, there are a total of 5 bond pairs and 2 lone pairs.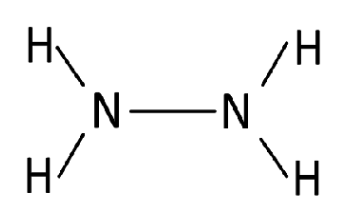
Hydrazine Production
Now, explore how hydrazine is produced through two different processes:
Pechiney-Ugine-Kuhlmann Process
In this method, ammonia (NH 3 ) and hydrogen peroxide (H 2 O 2 ) come together to create hydrazine (N 2 H 4 ) and water (H 2 O). The reaction looks like this:
2NH 3 + H 2 O 2 → N 2 H 2 + 2H 2 O
Raschig Process
In the Raschig process, alkaline ammonia mixed with water reacts with sodium hypochlorite (NaOCl) to produce hydrazine (N 2 H 4 ), sodium chloride (NaCl), and water (H 2 O). The reaction can be represented as:
2NH 3 + NaOCl → N 2 H 4 + NaCl + H 2 O
Hydrazine Formula Physical Properties
Chemical formula: N 2 H 4
Molecular weight: 32 g/mol
Density: 1.02 g/cm3
Odor similar to ammonia
Colorless, oily, fuming liquid
Refractive index: 1.46
Boiling point: 114°C
Melting point: 2°C
Hydrazine Formula Chemical Properties
When hydrazine reacts with acids and certain metallic salts, it produces compounds used in making specific explosives and fungicides. In reactions with organic compounds, hydrazine forms alkyl hydrazines. The combustion of hydrazine in the presence of oxygen results in the production of nitrogen gas and water. This is an exothermic process, and the reaction is as follows:N 2 H 4 + O 2 → N 2 + 2H 2 O
When hydrazine reacts with chloramine, it generates nitrogen gas and ammonium chloride. The reaction is as follows:N 2 H 4 + 2NH 2 Cl → 2NH 4 Cl + N 2
Hydrazine Harmful Effect
Hydrazine can be harmful if inhaled or if it comes into contact with the body. It can lead to problems like irritation in the respiratory tract, itchy eyes, and skin. To stay safe when working with hydrazine, it's important to follow these precautions: Wear protective gear like safety goggles, gloves, and a respirator when needed. Donning a safety suit and sturdy boots adds an extra layer of protection.
Uses of Hydrazine
Hydrazine finds various important uses:
Rocket Fuel: Hydrazine is utilized as a propellant in rockets and other spacecraft.
Reducing Agent: It serves as a reducing agent in chemical reactions.
Fungicides: Hydrazine is used in making agricultural fungicides, which help protect crops from fungal diseases.
Corrosion Prevention: It works as a corrosion-preventing agent in cooling water reactors, safeguarding equipment from rust and decay.
Nitrogen Production: On a smaller scale, hydrazine can be used to produce nitrogen gas.
Foaming Agent: It's also used as a foaming agent in the production of polymer foams, which have various applications in industries.
Blowing Agents: Hydrazine is used as a precursor in the production of blowing agents, which create gas bubbles in materials like foam.
| Related Link | |
| Hyposulfurous Acid Formula | Iodide Formula |
| Molybdic Acid Formula | Iron (II) Oxide Formula |
Hydrazine Formula FAQs
What is the chemical formula for hydrazine?
How does hydrazine decompose, and what does it produce?
What is the ratio of nitrogen (N) atoms to hydrogen (H) atoms in the hydrazine molecule?
What are the main uses of hydrazine?










