
Iron II Sulfate Formula: Iron, denoted by the chemical symbol Fe (derived from the Latin word "Ferrum"), holds the atomic number 26 and resides within the initial transition series of the periodic table, belonging to Group 8. This elemental substance predominantly appears in the shape of a robust, gray-colored metal Iron is used to make steel, and it's an important part of many tools, buildings, and vehicles. But in living things, like our bodies and the food we eat, we don't find much iron. It's like a giant iron gate that's locked up tight.
Iron II Sulfate Formula
Sulfate, a compound comprising sulfur and oxygen atoms, stands as one of the most prevalent naturally occurring minerals on Earth. Its existence is widespread across the environment, primarily stemming from atmospheric and terrestrial processes. The primary sources of sulfate are rocks and minerals harboring sulfides, sulfur emissions resulting from the erosion of evaporite deposits, and even volcanic activity. Sulfates manifest as salts containing various elements, including potassium, sodium, calcium, magnesium, and barium.
What is Iron II Sulfate Formula
Iron(II) sulfate, colloquially known as green vitriol, falls under the category of iron salts. It encompasses diverse salt compositions following the formula FeSO 4 · xH 2 O. Other designations for ferrous sulfate encompass Iron sulfate, green vitriol, iron vitriol, copper, melanterite, and Szomolno kit. The most widespread variant of this substance is the blue-green heptahydrate, characterized by a seven-water-molecule hydrate. All ferrous sulfate compounds exhibit uniform solubility in water, leading to the formation of the same aqua complex denoted as [Fe(H2O) 6 ] 2+ .Iron II Sulfate Formula Structure
This complex is paramagnetic and possesses an octahedral molecular structure. The terminology of "copper" harks back to an era when copper (II) sulfate was referred to as blue copper, distinguishing it from ferrous sulfate (green) and zinc sulfate (white). When iron filings are introduced into a copper sulfate solution, iron displaces copper due to its higher reactivity, ultimately leading to the production of ferrous sulfate. Notably, ferrous sulfate features on the World Health Organization's roster of essential medicines, recognized as the safest and most efficacious pharmaceutical necessary within the medical field.
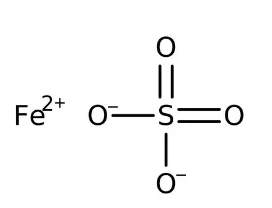
Iron II Sulfate Hexahydrate Formula
The formula for Iron(II) sulfate hexahydrate is FeSO 4 · 6H 2 O. This represents a hydrated form of iron(II) sulfate, meaning it contains six water molecules (H 2 O) associated with each formula unit of iron(II) sulfate.Iron II Sulfate Monohydrate Formula
The formula for Iron(II) sulfate monohydrate is FeSO 4 · H 2 O. This indicates that one water molecule (H 2 O) is associated with each formula unit of iron(II) sulfate.Production of Iron II Sulfate
One common method of producing Ferrous Sulfate is through the use of sulfuric acid pickling baths. These baths are employed to prepare steel sheets or bars for plating or coating processes, and they result in the formation of a significant amount of Ferrous Sulfate as a by-product.Fe + H 2 SO 4 ⟶ FeSO 4 + H 2
Another source of Ferrous Sulfate production involves the sulfate process, which is utilized in the production of titanium dioxide from ilmenite. Oxidation of pyrite can also yield Ferrous Sulfate, and this process is economically viable.2FeS 2 + 7O 2 + 2H 2 O ⟶ 2FeSO 4 + 2H 2 SO 4
Additionally, Ferrous Sulfate can be produced by displacing less reactive metals than iron from a sulfate solution.CuSO 4 + Fe ⟶ FeSO 4 + Cu
Iron II Sulfate Formula Properties
Iron(II) Sulfate, also known as Ferrous Sulfate, is a chemical compound with specific physical and chemical properties. This compound can be obtained through various methods, and it has a variety of applications in different industries.
Iron II Sulfate Formula Physical Properties
- Molar Mass of Anhydrous Ferrous Sulfate: 151.91 g/mol
- Color: White crystals (in Anhydrous form)
- Melting Point: 56°C – 64°C
- Boiling Point: Greater than 300°C
- Solubility: It is soluble in water
Iron II Sulfate Formula Chemical Properties
When Ferrous Sulfate reacts with aluminum, it forms aluminum sulfate and metallic iron in a displacement reaction.
2Al + 3FeSO 4 → Al 2 (SO 4 ) 3 + 3Fe
In the presence of sulfuric acid, Ferrous Sulfate reacts with potassium permanganate to form ferric sulfate, manganese sulfate, potassium sulfate, and water.
10FeSO 4 + 2KMnO 4 + 8H 2 SO 4 → 5Fe 2 (SO 4 ) 3 + 2MnSO 4 + 8H 2 O + K 2 SO 4
When Ferrous Sulfate is heated, it first loses its water of crystallization, and the green crystals transform into white anhydrous solids. Further heating causes the release of white smoke consisting of sulfur trioxide and sulfur dioxide, ultimately leaving behind reddish-brown iron oxide.
2 FeSO 4 → Fe 2 O 3 + SO 2 + SO 3
It's important to note that Iron Sulfate begins to decompose at approximately 680°C (1,256°F). These physical and chemical properties make Ferrous Sulfate a versatile compound with various industrial applications.
Applications of Iron (II) Sulfate
Commercial Use: In industrial settings, Ferrous Sulfate serves as a valuable precursor for the creation of various other iron compounds. Furthermore, it acts as a reducing agent and plays a crucial role in reducing chromate in cement, thereby converting it into less toxic Cr (III) substances. Over the centuries, Ferrous Sulfate has found application as a fixative in the textile industry for dyeing leather and as an ink source. The distillation of green vitriol (iron (II) sulfate) to treat sulfuric acid has been a known process for more than seven centuries.
Medicinal Use: Iron is a very important mineral found in some vitamins and mineral pills. It's used in medicine to help people who don't have enough iron in their bodies, which can lead to a condition called anemia. When you take the right amount of iron, it usually doesn't harm your liver. But if you take too much, it can be very toxic and even cause your liver to fail. There are different types of iron pills you can take by mouth, like iron fumarate, ferrous gluconate, and ferrous sulfate. Among these, ferrous sulfate is often seen as the safest and most affordable choice for iron supplements.
Beyond these uses, green vitriol, or Ferrous Sulfate, serves as a valuable reagent for detecting fungi and operates as an iron catalytic element in Fenton's reagent. Additionally, it stands as a crucial ingredient in gall ink, used for various writing and drawing purposes.
Moreover, Ferrous Sulfate plays an integral role in water purification, facilitating agglomeration to remove impurities, and is employed in the removal of phosphate in urban and industrial sewage treatment plants to prevent the eutrophication of water bodies. These diverse applications make Iron (II) Sulfate a versatile substance with significant importance in various industries.
| Related Link | |
| Hydrobromous Acid Formula | Hyposulfurous Acid Formula |
| Iodide Formula | Molybdic Acid Formula |
Iron II Sulfate Formula FAQs










