
Hydrogen Gas Formula: Hydrogen gas is composed of just two hydrogen atoms, making it the lightest element in group 1 of the periodic table. It is colorless, odorless, and tasteless, denoted by the symbol H with an atomic number of 1. Under standard conditions, hydrogen exists as a diatomic molecule with the formula H 2 .
This gas is not harmful or toxic, and it is highly flammable. It is considered a clean-burning fuel, produced from coal and water. Hydrogen gas is also known as Molecular Hydrogen. It can be stored as a compressed gas or a liquid and is used in the manufacturing of polyurethane plastics. It's also suitable for powering internal combustion engines in vehicles.
Hydrogen Gas Formula Molar Mass
The chemical formula for hydrogen gas is H 2 . Its molar mass, which is the sum of the atomic masses of its constituent atoms, is approximately 2.016 grams per mole.
Hydrogen Gas Formula Charge
Hydrogen gas, H 2 , is electrically neutral. This means it has no net electrical charge. Each hydrogen atom in the molecule has one proton and one electron, and when two hydrogen atoms combine to form H 2 , the total number of protons and electrons is equal, resulting in a neutral charge.
Hydrogen Gas Formula Diatomic
Hydrogen gas exists in a diatomic molecular form, which means it consists of two hydrogen atoms bonded together. Its chemical formula is H 2 . Each H 2 molecule contains two hydrogen atoms sharing electrons to form a stable molecule.
Preparation Hydrogen Gas
To create Hydrogen gas, you can react Zinc with Hydrochloric acid, resulting in Hydrogen gas and Zinc chloride:Zn + 2HCl → ZnCl 2 + H 2
Or, you can also make Hydrogen gas by combining Zinc with Sulphuric acid, which produces Zinc sulfate and Hydrogen gas:Zn + H 2 SO 4 → ZnSO 4 + H 2
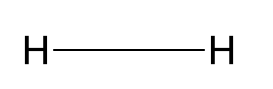
Hydrogen Gas Formula Structure
The structure of a hydrogen gas molecule (H 2 ) is straightforward. It consists of two hydrogen atoms bonded together. These two hydrogen atoms share electrons to form a covalent bond, creating a stable diatomic molecule. The structure is often represented as H-H, where the line between the two "H" symbols represents the covalent bond.
Hydrogen Gas Formula Properties
Hydrogen gas has several characteristics:
- Appearance: It is a colorless and odorless gas.
- Weight: Its molecular weight is 1.00794.
- Density: Hydrogen gas has a density of 0.0893 grams per liter (g/L).
- Melting Point: It melts at a very low temperature of -259.2 °C.
- Boiling Point: The boiling point of hydrogen gas is -252.9 °C.
- Solubility: It can dissolve in water, with a solubility of 1.62 mg/L at 21 °C.
Chemical Reactions of Hydrogen Gas
Hydrogen gas is involved in various chemical reactions: It reacts with nitrogen in the atmosphere to produce Ammonia:N 2 + 3H 2 → 2NH 3
In the production of Methanol, hydrogen gas is used and not separated from the synthesis gas stream:CO + 2H 2 → CH 3 OH
When hydrogen combines with chlorine, it forms Hydrogen Chloride (HCl):H 2 + Cl 2 → 2HCl
Hydrogen gas, under specific temperature and pressure conditions, can reduce metal oxides and metallic salts into metals:H 2 + FeO → Fe + H 2 O
It can also react with PdCl 2 to produce Palladium and Hydrogen Chloride:H2 + PdCl 2 → Pd + 2HCl
Practical Uses of Hydrogen Gas
Hydrogen gas has several important applications:
Weather Balloons: It's used to inflate weather balloons, usually combined with helium, to help gather information about the atmosphere for weather monitoring.
Car Engines: Hydrogen is used as a fuel in internal combustion engines, commonly found in cars, making vehicles run more cleanly and with reduced emissions.
Edible Oils Processing: In the food industry, hydrogen gas is utilized to transform edible vegetable oils into Vanaspati ghee, enhancing their texture and shelf life.
Polyurethane Plastics: Hydrogen gas plays a critical role in the production of polyurethane plastics, which are widely used in various products, such as foam insulation, cushioning materials, coatings, and adhesives
Welding and Metal Cutting: The intense heat from the oxy-hydrogen flame is valuable in industrial tasks like welding and cutting various metals with precision.
| Related Links | |
| Dichloroacetic Acid Formula | Tartaric Acid Formula |
| Dinitrogen Monoxide Formula | Sulfurous Acid Formula |
Hydrogen Gas Formula FAQs
What is the chemical formula for hydrogen gas?
What is the molar mass of hydrogen gas?
Is hydrogen gas electrically charged?
What is the structural formula of hydrogen gas?
What are the key properties of hydrogen gas?










