
Nitrogen Dioxide Formula: Nitrogen is a naturally occurring element essential for the growth and reproduction of both plants and animals. It's characterized as a chemical element with an atomic number of 7, comprising approximately 78 percent of Earth's atmosphere. This gas is colourless, odourless, and unreactive. When subjected to distillation from liquid air, it transforms into liquid nitrogen, a refrigerant with a boiling point of 77.4 Kelvin (195.8 °C). Nitrogen dioxide and manganese dioxide (MnO 2 ) are examples of oxides featuring two oxygen atoms, each directly bonded to an atom of another element, in this case, nitrogen (NO 2 ).
Also Check – Nucleophile Formula
Nitrogen Dioxide Formula
Nitrogen dioxide, a compound composed of nitrogen and oxygen, is categorized as one of several nitric oxides, including nitric oxide and nitrous oxide. Chemical formulas can be either empirical or molecular, with the empirical formula specifying the quantity of each element within a molecule and the molecular formula providing the exact count of atoms in each element. In the case of nitrogen dioxide, the empirical and molecular formula is NO 2 , denoting a 1:2 ratio of nitrogen to oxygen atoms. Nitrogen dioxide is also known as nitric oxide (IV) and is a significant air pollutant capable of absorbing UV light and causing skin cancer.
Structure of Nitrogen Dioxide
The covalent molecule nitrogen dioxide (NO 2 ) consists of a central nitrogen atom, a single bond to one oxygen atom, and a double bond to another oxygen atom.
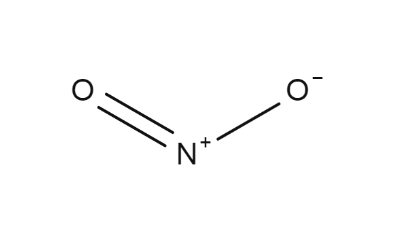
Physical Properties of Nitrogen Dioxide
- Chemical Formula: NO 2 (serving as both empirical and molecular formula)
- Molecular Weight: 46 g/mol (calculated as [(Molecular weight of Nitrogen * Number of Nitrogen atoms) + (Molecular weight of Oxygen * Number of Oxygen atoms)] = (14 g/mol * 1) + (16 g/mol * 2) = 14 + 32 = 46 g/mol.
- Density: 1.880 g/dm3
- Melting Point: −9.3 °C
- Boiling Point: 21.15 °C
Also Check – Tyndall Effect Formula
Chemical Properties of Nitrogen Dioxide
Thermal Properties: Nitrogen dioxide is in equilibrium with nitric oxide gas:
2 NO 2 ⇌ N 2 O 4 .
Oxidizing Agent: It acts as a potent oxidizing agent due to its weak N-O bond.
Hydrolysis Reaction: Hydrolysis yields nitrite and nitric acid:
2NO 2 (N 2 O 4 ) + H 2 O → HNO 2 + HNO 3 .
Rate of Reaction: The reaction rate is negligibly slow at low nitrogen dioxide concentrations.
Formation of Nitrites : Nitrites are formed from alkyl and metal iodides:
2CH 3 I + 2 NO 2 → 2CH 3 NO 2 + I 2
TiI 4 + 4NO 2 → Ti(NO) 4 + 2I 2
Also Check – Theoretical Yield Formula
Sources of Nitrogen Dioxide
The primary source of nitrogen dioxide emissions is combustion, contributing to nearly 98 percent of man-made NO emissions, particularly from stationary sources. Combustion-generated nitrogen oxides are primarily released as nitric oxide (NO), which rapidly transforms into the more harmful nitrogen dioxide in the atmosphere. Nitrogen dioxide negatively impacts human respiratory function, with prolonged exposure linked to an increase in respiratory illnesses. It also serves as a precursor to the formation of nitrate aerosols and nitrosamines, both of which have health implications. Due to its significant production and potential health and environmental impacts, nitrogen oxides are subject to stringent regulations and limits.
Also Check – Vapor Pressure Formula
Uses of Nitrogen Dioxide
Nitrogen dioxide serves a diverse range of applications, showcasing its versatility in various industries:
Synthesis of Nitric Acid: Nitrogen dioxide plays a pivotal role in the chemical synthesis of nitric acid, contributing to the production of this essential industrial chemical.
Component in Oxidized Cellulose Compounds: It functions as a crucial component in the manufacturing process of oxidized cellulose compounds, contributing to the development of materials with specific properties and applications.
Catalyst: Nitrogen dioxide exhibits catalytic properties, enabling it to facilitate chemical reactions by lowering the activation energy required, thus expediting various industrial processes.
Manufacturing of Sulfuric Acid: In the production of sulfuric acid, nitrogen dioxide is employed as an integral component, participating in the intricate chemical processes that yield this widely used and vital acid.
Rocket Propulsion as an Oxidizer: In the field of rocket propulsion, nitrogen dioxide finds application as an oxidizer, essential for the combustion reactions that enable spacecraft and rockets to achieve the thrust required for space travel.
Oxidant and Flour Bleaching Agent: Nitrogen dioxide serves as a potent oxidant, playing a critical role not only in chemical reactions but also in the food industry, where it is utilized for the purpose of flour bleaching, enhancing the visual appeal and quality of various food products.
Role in Explosives Production: Nitrogen dioxide is integral to the production of explosives, where it contributes to the formulation of highly energetic compounds used for a wide range of applications, including military and industrial uses.
Nitrogen Dioxide Formula FAQs
What is nitrogen dioxide?
Where is nitrogen dioxide found?
How is nitrogen dioxide used in industry?
Is nitrogen dioxide harmful to the environment?
What is the chemical formula of nitrogen dioxide?










