
Manganese II Chloride Formula : Manganese (Mn) is a chemical element with the atomic number 25 on the periodic table. It can be naturally found in various foods such as nuts, seeds, grains, tea, and green leafy vegetables. Manganese appears as a silvery-white metal known for its hardness and brittleness.
Chlorine (Cl), on the other hand, is a greenish-yellow gas with an atomic number of 17 in the periodic table. It emits a pungent odor and is detrimental to the environment, being 2-5 times denser than air. Among the halogens, chlorine is the second lightest element and possesses two stable isotopes.
Manganese(II) chloride, an inorganic compound that serves as a significant nutrient for many plants and animals. It plays a vital role in their biological processes. To understand more about Manganese(II) chloride, let's explore its formula, structure, and properties.
Manganese II Chloride Formula
The chemical formula for Manganese (II) Chloride comes in a few different forms:
- Anhydrous form: MnCl2
- Dihydrate form: MnCl2·2H2O
- Tetrahydrate form: MnCl2·4H2O
Manganese II Chloride Formula Structure
The structure of Manganese (II) Chloride (MnCl 2 ) can vary depending on its hydration state. Here's a brief overview of the structures for the anhydrous and dihydrate forms:
- Anhydrous Form (MnCl 2 )
In the anhydrous form of Manganese (II) Chloride, there are no water molecules associated with the compound. It consists of manganese (Mn) ions bonded to chloride (Cl) ions. The manganese ion has a 2+ charge, and each chloride ion has a 1- charge. In this form, MnCl 2 exists as a white, crystalline solid.
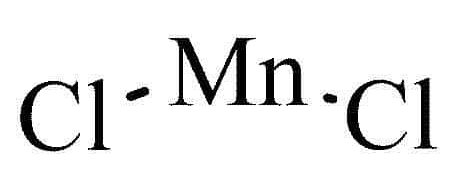
- Dihydrate Form (MnCl 2 ·2H 2 O)
The dihydrate form of Manganese (II) Chloride contains two water molecules (H 2 O) associated with each MnCl 2 unit. These water molecules are typically coordinated with the manganese ions. The structure involves the manganese ions surrounded by water molecules and chloride ions. In this hydrated form, MnCl 2 ·2H 2 O exists as a pale pink crystalline solid.
3.Tetrahydrate Form (MnCl2·4H2O)
In the tetrahydrate form of Manganese (II) Chloride (MnCl 2 ·4H 2 O), there are four water molecules associated with each MnCl 2 unit. The structure involves manganese ions surrounded by water molecules and chloride ions, resulting in a pale pink or light red crystalline solid. The presence of additional water molecules in this form affects its physical and chemical properties compared to the anhydrous and dihydrate forms.
Preparation of Manganese II Chloride
The laboratory preparation of Manganese (II) Chloride is achieved through various methods:
When Manganese metal reacts with HCl acid, Manganese (II) Chloride in tetrahydrate form is obtained:
Mn + 2HCl + 4H 2 O → MnCl 2 (H 2 O) 4 + H 2
By treating Manganese (II) Carbonate with HCl acid, Manganese (II) Chloride in tetrahydrate form can also be produced:
MnCO 3 + 2HCl + H 2 O → MnCl 2 (H 2 O) 4 + CO 2
Manganese (II) Chloride can be obtained by reacting Manganese(IV) oxide with a concentrated acid like HCl:
MnO 2 + 4HCl → MnCl 2 + 2H 2 O + Cl 2
Manganese II Chloride Formula Physical Properties
Manganese (II) Chloride comes in different forms with various physical properties:
Here are the physical properties of Manganese (II) Chloride in its different forms:
Anhydrous Form
- Molar weight: 125.844 g/mol
- Density: 2.977 g/cm3
- Melting point: 654 °C
Dihydrate Form
- Molar weight: 161.874 g/mol
- Density: 2.27 g/cm3
- Melting point: 135 °C
Tetrahydrate Form
- Molar weight: 197.91 g/mol
- Appearance: Pink solid
- Density: 2.01 g/cm3
- Melting point: 58 °C
Other Properties
- Boiling point: 1,225 °C
- Solubility: It dissolves in water, is slightly soluble in ethanol, but insoluble in ether.
Manganese II Chloride Formula Chemical Properties
Manganese (II) Chloride exhibits various chemical properties:
- When dissolved in water, it forms mildly acidic solutions with a pH of 4, containing the metal aquo complex [Mn(H 2 O) 6 ] 2+ . It acts as a weak Lewis acid.
- Manganese (II) Chloride can react with potassium carbonate (K 2 CO 3 ) to yield manganese carbonate (MnCO 3 ) and potassium chloride (KCl).
- In reactions with chloride ions, it can generate different solid compounds, including [MnCl 3 ] − , [MnCl 4 ] 2- , and [MnCl6] 4- .
- When exposed to typical organic ligands, it can undergo oxidation by air to form Mn(III) complexes, such as [Mn(CN) 6 ] 3 - and [Mn(acetylacetonate)3].
- Manganese (II) Chloride can also react with triphenylphosphine (Ph 3 P) to produce [MnCl 2 (Ph 3 P) 2 ] and with sodium cyclopentadienide (NaC 5 H 5 ) to form manganese cyclopentadienide (Mn(C 5 H 5 ) 2 ) and sodium chloride (NaCl) .
Applications of Manganese II Chloride
Manganese(II) Chloride finds various practical uses:
Leclanche Cell Batteries: It is employed in the construction of Leclanche cell batteries.
MRI Scanning: The paramagnetic properties of MnCl2 make it valuable as a contrast agent in MRI scanning, aiding in medical imaging.
P-NMR (Phosphorus Nuclear Magnetic Resonance): Aqueous solutions of this compound are utilized in P-NMR.
Dimension Determination: Manganese chloride is used in processes to determine dimensions.
| Related Link | |
| Magnesium Sulfate formula | Lithium Hydroxide Formula |
| Magnesium Oxide Formula | Lithium Oxide Formula |
Manganese II Chloride Formula FAQs
What is the formula for Manganese II Chloride?
What is the structure of the anhydrous form of Manganese II Chloride?
Describe the dihydrate form of Manganese II Chloride.
What characterizes the tetrahydrate form of Manganese II Chloride?
What are some common applications of Manganese II Chloride?










