
Methane formula is CH4 (one carbon atom and four hydrogen atoms), is a hydride of group-14 and is known as the simplest alkane. Its prevalence on Earth makes it a valuable fuel source, but its gaseous state poses challenges for extraction and storage under standard temperature and pressure. This colorless, odorless gas is abundant in nature and also produced by human activities. Methane Formula it is considered the simplest hydrocarbon. It a fundamental compound in the world of chemistry and a primary component of natural gas, has a straightforward molecular formula.
Methane Formula
The chemical formula of methane is CH₄. It represents the elemental composition of methane, breaking down as follows: C: Stands for carbon, with one atom of carbon. H₄: Denotes hydrogen, with four atoms of hydrogen. This composition showcases the simplicity of methane, the smallest and simplest hydrocarbon in the alkane family.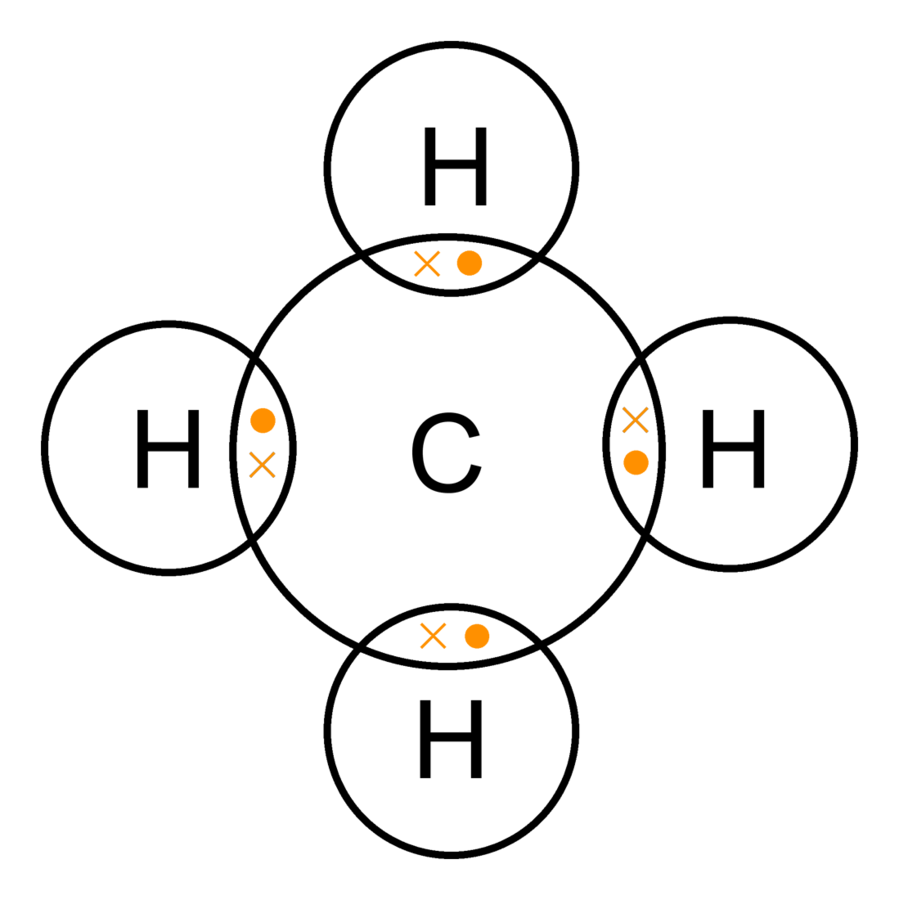
Also Read: Butane Formula
Methane Formula Structure
Methane's molecular structure is tetrahedral, with the carbon atom at the center bonded to four hydrogen atoms. This structure results from the carbon atom forming four single covalent bonds with the four hydrogen atoms. The tetrahedral arrangement is due to the carbon atom's hybridization of its orbitals, specifically sp³ hybridization. This arrangement minimizes electron repulsion and stabilizes the molecule. The four hydrogen atoms are evenly distributed around the carbon atom, creating a symmetrical and highly stable molecule. This structure is responsible for methane's physical and chemical properties, making it a vital component in various industrial and energy applications.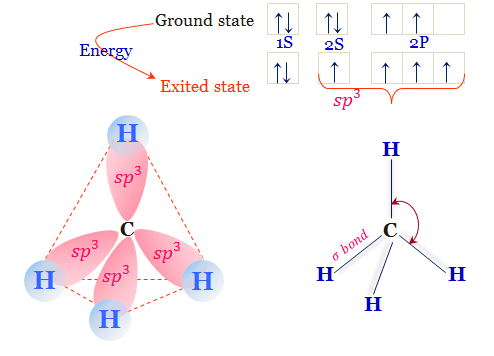
Methane Formula and Valency
Valency refers to the number of bonds an element can form with other elements. In the methane formula (CH₄), carbon has a valency of four, which means it can form up to four covalent bonds. In methane, carbon fully utilizes its valency by bonding with four hydrogen atoms. This leads to the formation of strong covalent bonds, resulting in a stable molecule. Understanding the valency of elements is crucial in predicting their chemical behavior and how they form compounds.Also Read: Thiourea formula
Methane Formula Molar Mass
The molar mass of methane can be calculated by summing the atomic masses of its constituent atoms. The molar mass is essential for various chemical calculations, including stoichiometry and gas laws. Here's how to calculate the molar mass of methane: - Carbon (C) has an atomic mass of approximately 12.01 g/mol. - Hydrogen (H) has an atomic mass of approximately 1.01 g/mol. To find the molar mass of methane (CH₄): Molar mass of CH₄ = (Number of C atoms × Atomic mass of C) + (Number of H atoms × Atomic mass of H) Molar mass of CH₄ = (1 × 12.01 g/mol) + (4 × 1.01 g/mol) = 16.05 g/mol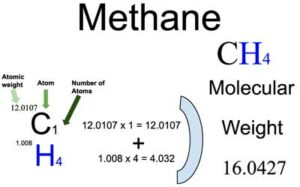 The molar mass of methane is approximately 16.05 grams per mole (g/mol). This value is essential for determining the amount of methane in a given sample or calculating its volume and behavior under specific conditions, such as in the Ideal Gas Law.
The molar mass of methane is approximately 16.05 grams per mole (g/mol). This value is essential for determining the amount of methane in a given sample or calculating its volume and behavior under specific conditions, such as in the Ideal Gas Law.
CH 4 Uses (Methane)
- The fuel is used in automobiles, ovens, and water heaters
- Electricity is generated by it
- In its refined liquid form, it is used as rocket fuel
- In industries, it is used as an antifreeze ingredient
- Fertilizers often contain it
- Products are sanitized with it
- Gas-fired power stations use it
- Gas cookers use it
- Gas appliances are tested with it
Properties of Methane
Pure methane is an energy-rich feedstock with an energy density of 55.7 MJ/kg that can be used for the generation of electricity, for domestic heating, and for cooking. Below is enlist a few properties of Methane| Properties of Methane | |
| CH 4 | Methane |
| Molecular Weight/ Molar Mass | 16.04 g/mol |
| Density | 0.656 kg/m³ |
| Boiling Point | −161.50 °C |
| Melting Point | −182.5 °C |
Methane Formula Molecular
Methane, also known as marsh gas, fossil fuel, carbon tetrahydride, and hydrogen carbide, is a tetrahedral molecule composed of four identical C-H bonds. Its electronic structure is described by four bonding molecular orbitals (MOs) resulting from the overlap of valence orbitals on carbon and hydrogen. The lowest-energy MO is formed from the combination of the 2s orbital on carbon with the 1s orbitals on the four hydrogen atoms in-phase. With its abundance on Earth, methane serves as a beautiful fuel but poses challenges in capturing and storing it under normal temperature and pressure conditions. It has a molar mass of 16.043 g·mol−1.| Related Links | |
| Calcium Bromide Formula | Carbon Monoxide Formula |
| Calcium Iodide Formula | Chloroacetic Acid Formula |
Methane Formula FAQs
Is methane CH₃ or CH₄?
Methane is represented by the chemical formula CH₄, not CH₃. The formula CH₄ signifies that methane consists of one carbon (C) atom bonded to four hydrogen (H) atoms.
Is CH₄ a methane?
Yes, CH₄ is indeed the chemical formula for methane. It is the correct and widely accepted representation of methane's elemental composition.
Which is CH₄ gas?
CH₄ is the chemical formula for methane gas. Methane is a colorless, odorless, and highly flammable gas that is the primary component of natural gas. It is commonly used as a fuel for heating, electricity generation, and as a feedstock in various industrial processes.
Why is CH₄ a gas?
Methane (CH₄) is a gas at standard temperature and pressure (STP), which is defined as 0 degrees Celsius (32 degrees Fahrenheit) and 1 atmosphere of pressure (approximately 101.3 kPa). The gaseous state of methane is primarily due to its relatively low molecular weight and weak intermolecular forces between its molecules. The small size of the methane molecule and the absence of polar bonds lead to limited attractive forces between molecules, allowing them to move freely and exist as a gas under normal conditions.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









