
Monatomic gases formula is a gas composed of single-atom molecules, such as helium or sodium vapor. These are distinct from polyatomic gases containing two or more atoms per molecule. The thermodynamic behaviour of a monatomic gas at ordinary temperatures is elementary, as it lacks the rotational and vibrational components that characterize their polyatomic counterparts. As such, its heat capacity does not depend on temperature or molecular/atomic weight, whilst its entropy (a measure of disorder) is determined solely by temperature and molecular weight.
Monoatomic Gas Examples
A perfect or ideal gas follows the general gas law. This is a generalized form of Boyle's and Charles's laws, which states that the product of pressure (P) multiplied by volume (V), is proportional to absolute temperature (T). In equation form this is PV = kT, where k is a constant. This relation expresses the equation of state for the substance and more than adequately describes its behavior. Based on the kinetic theory of gases, the general law of gases can be derived -- The gas consists of many molecules that are in random motion and obey Newton's laws.
- Compared to the volume of the gas, the molecules occupy a negligible amount of space. There are no forces at work.
Also Read: Molar Volume Formula
From this, the behavior of real gases is described quite accurately by the general gas law at elevated temperatures. However, it does not obey the equation when they are near their condensation point - the temperature at which they liquefy. To make the general law applicable to any gas, Avogadro's law is utilized and the constant for specifying the amount of gas is expressed using gram-moles and molecular weight in grams. As a result, we get the equation of state pv/t = nR, where R refers to the universal gas constant.Noble Gases are Monatomic
"Monatomic", formed from the words "mono" and "atomic", refers to single atoms. It is usually used for certain gases, such as the noble gases of argon, krypton, and xenon, typically found at standard conditions. Any chemical element may be present in a monatomic form in its gas phase at high temperatures. For this reason, the thermodynamic behavior of monatomic gases is generally much simpler than other more complex polyatomic gases because rotational or vibrational energy does not come into play.Also Read - Malic Acid Formula
The noble gases are single-atom molecules that, at standard temperature and pressure (STP), hold a full outer valence shell, making them relatively non-reactive. Historically described as inert elements, only helium, and neon have yet to form chemical compounds. However, when grouped together with homonuclear diatomic gases like nitrogen (N2), they are known as “elemental” or “molecular” gases; distinct from molecules of compounds.What is Monatomic Gas?
It is a combination of mono and atomic which generally means one atom. It is used in both Physics and Chemistry to refer to gases as monatomic gases. At sufficiently high temperatures, all the chemical elements that are monatomic gases are present in the gaseous phase, which we already know.Also Check - Iron (III) Hydroxide Formula
The noble gases are monoatomic, which means that they are non-reactive. These glasses can be used for applications such as:- Due to its lower density than air, helium fills balloons.
- When electricity flows through gas neon, it glows and is used for advertising signs.
- In a light bulb, argon prevents filament burning since it is unreactive.
- Diatomic molecules are molecules composed of only two atoms.
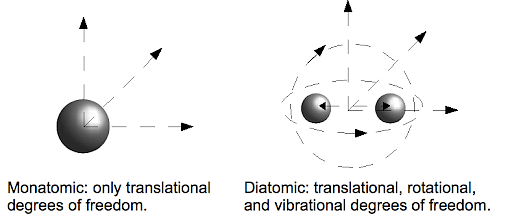
What are Diatomic Molecules?
Suppose a diatomic molecule is composed of only two atoms. In that case, it is called a homonuclear molecule, and if it is composed of two elements, it is called a heteronuclear molecule.Monoatomic Gases Formula FAQs
What are monatomic gases?
Monatomic gases are elements in gaseous form with single atoms as their fundamental units, such as helium (He) and neon (Ne).
What is the specific heat capacity formula for monatomic gases?
The specific heat capacity (Cv) of monatomic gases is given by Cv = (3/2)R, where R is the gas constant.
Why do monatomic gases have a specific heat capacity of (3/2)R?
Monatomic gases have only translational kinetic energy modes, and the equipartition theorem dictates that each mode contributes (1/2)R to the specific heat, resulting in (3/2)R for three translational modes.
Which gases are considered monatomic in the ideal gas law?
Noble gases like helium (He), neon (Ne), argon (Ar), krypton (Kr), and xenon (Xe) are typically considered monatomic in the ideal gas law.
How does the behavior of monatomic gases differ from diatomic or polyatomic gases?
Monatomic gases have simpler energy distributions and higher specific heat capacities than diatomic or polyatomic gases because they lack rotational and vibrational modes of motion.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









