
The phthalic acid formula is a significant organic compound with the chemical formula C8H6O4. This chemical structure consists of eight carbon atoms (C), six hydrogen atoms (H), and four oxygen atoms (O). Its distinctive molecular arrangement sets phthalic acid apart, featuring two carboxylic acid groups (-COOH) attached to a benzene ring.
The phthalic acid formula and its derivatives have various industrial applications. Most notably, phthalic anhydride, derived from phthalic acid, is a crucial starting material for producing phthalate plasticizers. These plasticizers are incorporated into polymers, especially polyvinyl chloride (PVC), to enhance their flexibility and durability. They are commonly used in vinyl flooring, cables, and automotive interiors.Phthalic Acid Formula and Charge
Phthalic acid is an essential organic compound with the molecular formula C8H6O4. It consists of eight carbon atoms (C), six hydrogen atoms (H), and four oxygen atoms (O). Its chemical structure contains two carboxylic acid groups (-COOH) attached to a benzene ring. These carboxylic groups are responsible for its acidic properties.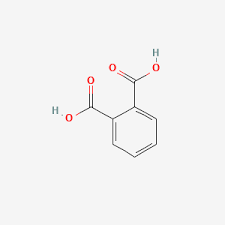 Phthalic acid has no net charge under normal conditions, as it is a neutral molecule. However, the carboxyl groups can dissociate in aqueous solutions to release hydrogen ions (H+), making it weakly acidic.
Phthalic acid has no net charge under normal conditions, as it is a neutral molecule. However, the carboxyl groups can dissociate in aqueous solutions to release hydrogen ions (H+), making it weakly acidic.
Also Read : Caffiene Chemical Formula
Phthalic Acid Formulation
Phthalic acid is synthesized through the oxidation of naphthalene or orthoxylene, two aromatic hydrocarbons. The oxidation process typically employs a strong oxidizing agent, such as potassium permanganate (KMnO4), in the presence of sulfuric acid (H2SO4). The resulting product is phthalic acid, which can be further processed into various derivatives. The reaction generally proceeds as follows: 1. Oxidation of naphthalene: C10H8 + 4 KMnO4 + 5 H2SO4 → 4 COOH(C6H4)COOH + 4 K2SO4 + 5 H2O 2. Oxidation of orthoxylene: 2 C6H4(CH3)2 + 3 O2 → 2 COOH(C6H4)COOH + 2 H2O The resulting product is phthalic acid (1,2-benzenedicarboxylic acid). Phthalic acid can then be further processed into various derivatives for different industrial applications, including the production of plasticizers, dyes, pharmaceuticals, and more.Also Read : Butane Formula
Phthalic Acid Formula and Uses
Phthalic acid and its derivatives find widespread use in several industries: Plastics Industry: Phthalic anhydride, derived from phthalic acid, is a key precursor for producing phthalate plasticizers. These plasticizers are added to polyvinyl chloride (PVC) and other polymers to improve flexibility and durability. They are commonly used in the manufacture of products like vinyl flooring, cables, and automotive interiors. Dye and Pigment Manufacturing: Phthalic acid is used to synthesize various dyes and pigments, including phthalein dyes, which are often used as pH indicators. Pharmaceuticals: It is utilized to prepare certain pharmaceuticals and pharmaceutical intermediates. Resin Production: Phthalic acid is employed to manufacture alkyd resins, which are widely used in paints, coatings, and varnishes. Laboratory Reagent : In laboratories, phthalic acid may be used as a reagent in chemical analyses. Textile Industry: It is used in the textile industry for dyeing and printing fabrics.Also Read : Acetylene Formula
Phthalic Acid Formula Weight
The molecular weight of phthalic acid can be calculated by adding the atomic weights of its constituent elements. Carbon (C) has an atomic weight of approximately 12.01 g/mol, hydrogen (H) has an atomic weight of approximately 1.01 g/mol, and oxygen (O) has an atomic weight of approximately 16.00 g/mol. Using the formula C8H6O4, we can calculate the molecular weight as follows: Molecular weight of phthalic acid (C8H6O4) = (8 × 12.01 g/mol) + (6 × 1.01 g/mol) + (4 × 16.00 g/mol) = 166.08 g/mol The molecular weight of phthalic acid is approximately 166.08 grams per mole. Phthalic acid formula, with its distinct molecular formula C8H6O4, is a versatile compound with numerous applications in the chemical, plastics, and textile industries. Its formulation involves the oxidation of aromatic hydrocarbons, and it is used for its acidic properties as a precursor in plastics manufacturing and in the production of various chemical products. Understanding its molecular structure, change behavior, and diverse uses is essential for its application in various industrial processes.| Related Links | |
| Galactose Formula | Formic acid Formula |
| Formaldehyde Formula | Formic acid Formula |
Phthalic acid formula FAQs
What is the chemical name of phthalic acid?
The chemical name of phthalic acid is indeed "phthalic acid." It is also known by its systematic IUPAC name, 1,2-benzenedicarboxylic acid.
What is the structure of phthalic acid?
Phthalic acid has a chemical structure composed of a benzene ring with two carboxylic acid groups (-COOH) attached at positions 1 and 2 on the benzene ring. This distinctive structure is responsible for its properties and applications in various industries.
What is phthalic acid used in?
Phthalic acid and its derivatives have several industrial applications. It is a precursor for producing phthalate plasticizers used to enhance the flexibility of polymers like PVC. Additionally, phthalic acid is used in the dye and pigment industry, pharmaceutical manufacturing, production of alkyd resins for paints, and as a reagent in chemical analyses.
What is the formula for phthalic acid in Class 12?
In a Class 12 chemistry context, when referring to the formula for phthalic acid, it is typically denoted as C8H6O4. This formula represents the molecular composition of phthalic acid and is fundamental in understanding its chemical properties and reactions at an advanced level of chemistry education.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









