Potassium Nitrate Formula: Potassium, denoted by the symbol K and possessing an atomic number of 19, exhibits an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 . Positioned in the fourth period and belonging to the first group of the periodic table, potassium plays a crucial role in promoting plant growth. Its primary application lies in the form of potassium chloride (KCL), widely Uses in the manufacture of fertilizers.
Nitrogen, symbolized as N and characterized by an atomic number of 7, displays an electron configuration of 1s 2 2s 2 2p 3 . It is a colorless, odorless, and tasteless element and holds the distinction of being the most abundant constituent of Earth's atmosphere, constituting approximately 78% of the atmosphere.
Oxygen, represented by the symbol O and bearing an atomic number of 8, features an electron configuration of 1s 2 2s 2 2p 4 . Classified within the chalcogen group of the periodic table, oxygen is a highly reactive nonmetal and stands as the most prevalent element on our planet. Its primary applications include contributions to the production of plastics, steel, and glass.
Potassium Nitrate Formula
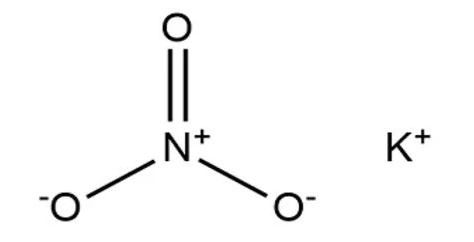
Potassium nitrate, also referred to as saltpeter or niter, is a chemical compound with the formula KNO 3 , consisting of potassium, nitrogen, and oxygen. This white crystalline substance is well-known for its potent oxidizing properties and is used in fertilizers as a source of both nitrogen and potassium. Its solubility in water is moderate, and it becomes more soluble as the temperature increases. In 1270, Syrian chemist Hasan al-Rammah documented a method for purifying and obtaining refined potassium nitrate from saltpeter.
Potassium Nitrate Formula Physical Properties
Potassium Nitrate is represented by the chemical formula KNO 3 .
It presents itself as a solid, white crystalline substance.
The crystal structure of potassium nitrate is classified as Aragonite, which is Orthorhombic in nature.
Its molar mass is calculated to be approximately 101.1032 g/mol.
The density of potassium nitrate is measured at 2.109 g/cm3.
It has a melting point of 334°C.
The boiling point of potassium nitrate is 400°C.
Potassium Nitrate Formula Chemical Properties
Potassium Nitrate is denoted by the chemical formula KNO 3 .
It exhibits moderate solubility in water, with an increase in solubility observed as the temperature rises.
Due to its oxygen , nitrate serves as a potent oxidizing agent.
When reacted with hydrochloric acid, Potassium Nitrate yields nitric acid. This can be achieved by introducing concentrated sulfuric acid to dry Potassium Nitrate, resulting in an overheating reaction that allows for the distillation of nitric acid.
Potassium Nitrate combines with sulfur and charcoal to produce gunpowder, also known as black powder.
Uses of Potassium Nitrate
Potassium nitrate's most famous role is as an oxidizer in black powder, which historically powered firearms. While it's been replaced by smokeless powders for firearms, it continues to find use in black powder rocket engines, fireworks, and even tobacco combustion in cigarettes.
Potassium Nitrate plays an important role in the production of fertilizers, providing a source of both nitrogen and potassium for plant growth.
It serves as an effective nitrate source in various applications.
In the realm of explosives, it acts as an oxidizer in black powder, which is used in rocket motors and fireworks.
This versatile compound finds its way into certain food applications, where it contributes to thickening soups and other culinary uses.
Additionally, Potassium Nitrate is an ingredient in some toothpaste formulations designed for individuals with sensitive teeth.
In the medical field, it has been historically utilized in asthma treatment.
It also serves as an electrolyte in salt bridges and plays a crucial role in condensed aerosol fire suppression systems as an active component.
| Related Links | |
| Potassium Bromate Formula | Gold Formula |
| Phosphate Formula | Helium Formula |
Potassium Nitrate Formula FAQs
What's the formula for Potassium Nitrate?
Where do we see Potassium Nitrate being used?
What is the structure of Potassium Nitrate's crystals?
What is the real-world application of KNO3?