
Sodium Chlorate Formula: Sodium chlorate is an inorganic compound represented by the chemical formula NaClO₃. This substance appears as a white crystalline powder. It is readily dissolvable in water, and exhibits hygroscopic properties. At temperatures exceeding 300°C. it undergoes decomposition, releasing oxygen and resulting in the formation of sodium chloride.
Sodium and chlorate combine to create sodium chlorate. Sodium, characterized by its reactivity, soft metallic nature, and low melting point, holds a paramount position among all alkali metals in commercial applications. At temperatures above 200°C, sodium undergoes a reaction with hydrogen to produce sodium hydride. Sodium is recognized by its atomic number 11 and is symbolized as Na on the periodic table.
The chlorate anion is represented by the chemical formula ClO 3 – , with the chlorine atom exhibiting a +5 oxidation state. The word "chlorate" can refer to any compound containing this anion. Essentially, chlorate is a salt originating from perchloric acid.
Sodium Chlorate
Sodium chlorate, with the chemical formula NaClO₃, is an inorganic compound known for its white crystalline structure that readily dissolves in water. It exhibits natural hygroscopic properties, absorbing moisture from the environment, and at temperatures above 573 Kelvin, it decomposes to release oxygen (O 2 ) and produce sodium chloride. Large quantities of sodium chlorate are annually manufactured, primarily through the oxidation of certain materials, for the production of high-quality paper used for various applications.Sodium Chlorate Formula
Sodium chlorate is an inorganic compound with the formula NaClO₃. It presents as a white crystalline powder with hygroscopic qualities and can be generated by electrolysis of a heated sodium chloride solution. Another name for sodium chlorate is sodium chlorate (V). In this brief article, we will discuss the sodium chlorate formula, its characteristics, chemical structure, and applications.
Sodium Chlorate Formula and Structure
Sodium chlorate, with a chemical formula of NaClO 3 and a molar mass of 106.44 g/mol, exists in the form of hygroscopic, white crystalline solid cubes. At approximately 300°C, it undergoes a gradual decomposition, yielding oxygen and salts. Its chemical structure is represented as follows: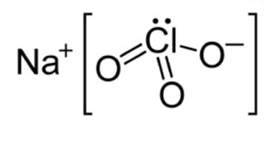
Sodium Chlorate Preparation
Sodium chlorate is produced through the electrolysis of a standard brine solution containing sodium chloride and water, with its chemical formula as follows: NaCl + 3H 2 O + 6e → NaClO 3 + 3H 2 . The specific procedure can vary based on adjustments in pH and temperature. The preparation of sodium chlorate is a straightforward process. Chlorine gas accumulates at the anode, while hydrogen gas (H 2 ) collects at the cathode. At this point, chlorine undergoes hydrolysis within the cell, forming anionic hypochlorite groups and resulting in the creation of sodium chlorate. These sodium chlorate particles manifest in crystalline form. The solution obtained after hydrolysis is often referred to as a "cell solution." Subsequently, this solution is extracted from the apparatus. The resulting crystals are washed, dried, and then stored in a dry environment. Depending on its intended application, sodium chlorate can be marketed in the form of either crystals or liquid.Sodium Chlorate Formula Physical Properties
Sodium chlorate exhibits a color range from bright yellow to white in its crystalline solid form. It is soluble in water and possesses a higher density than water, allowing it to quickly sink and dissolve. When it comes into contact with water, sodium chlorate can trigger highly exothermic reactions, potentially leading to intense fires, even when only 30% of the molecules are submerged.
The density of sodium chlorate is measured at 2.49 g/cm. It has a boiling point of 300°C and a melting point of 248°C. Additionally, it is soluble in certain organic solvents like glycerol and methanol, with slight solubility in acetone.
Sodium Chlorate Formula Chemical Properties
Sodium chlorate is a potent oxidizing agent, thanks to the presence of hypochlorite ions, which facilitate the oxidation and bleaching of various substances.
When sodium chlorate reacts with potassium bromide and hydrochloric acid, it yields products in the form of potassium chloride, sodium chloride, bromine, and water:
NaClO 3 + 6KBr + 6HCl → 6KCl + NaCl + 3H 2 O + 3Br 2
Similarly, a chemical reaction with potassium iodide and hydrochloric acid results in the formation of sodium chloride and potassium chloride:
NaClO 3 + 6KI + 6HCl → NaCl + 3I 2 + 3H 2 O + 6KCl
Sodium chlorate can also engage in reactions with various chemicals, including other bromides and acids. It's important to note that close contact with materials such as wood, sulfuric acid, various metals, and synthetic substances can potentially lead to fires or explosions.
Due to its strong oxidizing properties, sodium chlorate is considered explosive. It should be stored separately and handled within a controlled environment to ensure safety.
Uses of Sodium Chlorate
Sodium chlorate finds a multitude of uses, including:
Herbicides, Explosives, and Pyrotechnics: It serves as a key ingredient in the production of herbicides, explosives, and pyrotechnics.
Paints, Inks, and Cosmetics: Sodium chlorate is utilized in the formulation of paints, inks, and cosmetics.
Pharmaceuticals: It plays a role in pharmaceutical applications.
Solvay Process: Sodium chlorate is involved in the Solvay process, which combines salt with H2SO4 and CH3OH as a reducing agent.
Dye Manufacturing: It acts as an oxidizing agent in large-scale dye manufacturing processes, aiding in the production of dyes.
Oxidizing and Bleaching: Sodium chlorate is used as an oxidizing and bleaching agent in various industrial applications.
Fertilizer and Explosives: It plays a role in the production of fertilizers and explosives.
Defoliants: It is used as a defoliant in agriculture.
Paper and Leather: Sodium chlorate is utilized in the papermaking process and in tanning calfskins.
Pulp Coloring: It is used as a colored pulp in the paper manufacturing industry.
These diverse applications highlight the versatility and importance of sodium chlorate in various industries.
| Related Links | |
| Selenous Acid Formula | Pyrophosphoric Acid Formula |
| Potassium Sulfite Formula | Dinitrogen Pentoxide Formula |
Sodium Chlorate Formula FAQs
What is the chemical formula of sodium chlorate?
What is the appearance of sodium chlorate?
How does sodium chlorate decompose at high temperatures?
What is the atomic number of sodium?
What is the chemical structure of sodium chlorate?










