
Sodium Fluoride Formula: Sodium fluoride formula is NaF, It is an inorganic compound with the chemical formula NaF. This substance exists as a colorless or white solid and readily dissolves in water. It finds application in small quantities for water fluoridation, which helps prevent tooth decay.
Additionally, it is used in toothpaste and various topical pharmaceutical products for the same purpose. Sodium fluoride also had a significant number of prescriptions in the United States in 2020, ranking as the 265th most commonly prescribed medication with over 1 million prescriptions. Furthermore, it plays a role in metallurgy and medical imaging.
Sodium, denoted by the chemical symbol Na (derived from Latin natrium), is an elemental substance distinguished by an atomic number of 11. It exhibits a delicate, silvery-white appearance and boasts a high level of reactivity. Sodium is classified within group 1 of the periodic table as an alkali metal. Its lone stable isotope is 23Na. In its free metallic form, sodium does not occur naturally, necessitating its production from compounds.
Sodium ranks as the sixth most abundant element in the Earth's crust and is commonly found in various minerals such as feldspars, sodalite, and rock salt (sodium chloride, NaCl). Notably, numerous sodium salts display high water solubility, owing to the leaching of sodium ions from Earth's minerals due to the influence of water. In fact, sodium and chlorine stand out as the most prevalent dissolved elements in the Earth's oceans when measured by weight.
Sodium Fluoride Formula
Sodium Fluoride formula is NaF. It serves various applications in trace amounts. It finds use in drinking water fluoridation, toothpaste, and metallurgy. When sodium fluoride solutions come into contact with hard water, they can precipitate insoluble compounds of calcium and magnesium fluoride. In the context of water fluoridation, this compound is typically used in its dry form, requiring manual weighing and addition to the mixing tank.
A mineral variety of NaF known as Villiaumite exists, albeit relatively rarely. It has been identified in plutonic nepheline syenite rock formations. Villiaumite exhibits limited solubility in hydrofluoric acid (HF) and ammonia, but it is not soluble in alcohol, acetone, sulfur dioxide (SO 2 ), or dimethylformamide.
Sodium Fluoride Formula Structure
The composition of Sodium Fluoride consists of one fluoride anion (F – ) and one sodium cation (Na + ), resulting in its chemical formula NaF. It possesses a molecular weight of 41.98 g/mol and adopts a cubic crystalline structure, akin to sodium chloride. In this structure, both Na + and F - ions occupy octahedral coordination sites, although its lattice spacing, measuring 462 pm, is slightly smaller than that of sodium chloride.
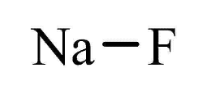
Sodium Fluoride Formula Physical Properties
It exhibits a density of 2.558 g/cm3.
The melting point of Sodium Fluoride occurs at 993°C.
It reaches a boiling point at 1704°C.
It has a standard molar entropy of 144 J·mol −1 K −1 .
The heat capacity of Sodium Fluoride is 46.82 J/(mol K).
Its solubility in water is 36.4 g/L at 0°C, 40.4 g/L at 20°C, and 50.5 g/L at 100°C.
Sodium Fluoride is classified as a basic substance, with a pH value of 8.
The refractive index of Sodium Fluoride is measured at 1.3252.
It exhibits a magnetic susceptibility of −16.4 × 10 −6 cm 3 /mol.
The vapor pressure of Sodium Fluoride reaches 1 mmHg at 1077°C.
The molecular structure of Sodium Fluoride is octahedral, while its crystal structure is cubic.
The standard enthalpy of formation for Sodium Fluoride is −573.6 kJ·mol −1 .
Sodium Fluoride Formula Chemical Properties
Sodium Fluoride formula is NaF.
When sodium fluoride interacts with water, it leads to the formation of hydrogen fluoride and sodium hydroxide. The chemical equation representing this reaction is as follows:
HF + NaOH → NaF + H 2 O
Additionally, sodium fluoride partakes in a displacement reaction with chlorine, yielding sodium chloride and fluorine. The chemical equation for this process is as follows:
NaF + Cl 2 → NaCl + F 2
Uses of Sodium Fluoride
Sodium fluoride serves a variety of purposes:
Pesticide Use: Sodium fluoride finds application as a pesticide, although it hasn't shown efficacy in preventing tooth decay in children when taken systemically during pregnancy.
Water Fluoridation: It is Used in the process of water fluoridation, which contributes to water purification and dental health.
Desilylation in Organic Synthesis: Similar to other fluorides, it is Used in organic synthesis, specifically for desilylation.
Finkelstein Reaction: Sodium fluoride is utilized in the Finkelstein reaction to generate fluorocarbons from sodium fluoride.
Laundry Industry Cleaning: It is used as a cleaning agent in the laundry industry.
Nuclear and Molten Salt Reactor Industries: Sodium fluoride has significant applications in the nuclear and molten salt reactor industries, contributing to various processes and technologies within these fields.
Treatment of Encephalopathies: Sodium fluoride L-glutamic acid monosodium salt, a compound derived from sodium fluoride, is used to treat encephalopathies associated with liver disorders.
Chemical Synthesis and Metallurgy: Sodium fluoride plays a vital role in specific chemical processes within synthesis and extractive metallurgy.
Reactivity with Electrophilic Chlorides: It reacts with electrophilic chlorides, including acyl chlorides, sulfur chlorides, and phosphorus chlorides.
| Related Links | |
| Potassium Fluoride Formula | Potassium Chlorate Formula |
| Potassium Bromate Formula | Gold Formula |
Sodium Fluoride Formula FAQs
What is the molecular formula for Sodium Fluoride?
What is the molecular weight of Sodium Fluoride?
What are the physical properties of Sodium Fluoride?
What is the crystal structure of Sodium Fluoride?
What are the chemical properties of Sodium Fluoride when it reacts with water?










