
Sodium Hydrogen Tartrate Formula: Sodium hydrogen tartrate is a salt frequently used as both an acidity regulator and a buffering agent. Studies have revealed its therapeutic potential in managing bowel diseases and its positive impact on locomotor activity.
Sodium Hydrogen Tartrate formula is C 4 H 5 O 6 Na.. Sodium Hydrogen Tartrate, also known as monosodium salt, tartaric acid, and sodium bitartrate. Its IUPAC name is Sodium 3-carboxy-2,3-dihydroxypropionate. This compound exhibits chemical stability when exposed to high temperatures, making it a valuable ingredient in the culinary world. Its non-reactive nature prevents it from interacting with metals and other substances.
Sodium Hydrogen Tartrate formula is C 4 H 5 O 6 Na. Sodium Hydrogen Tartrate serves as an effective buffering agent and is Used to acidify foods, preserving them and preventing rancidity or spoilage. Additionally, it boasts antioxidant properties, allowing it to function as a radical scavenger, targeting highly reactive species such as hydroxyl radicals and superoxide radicals.
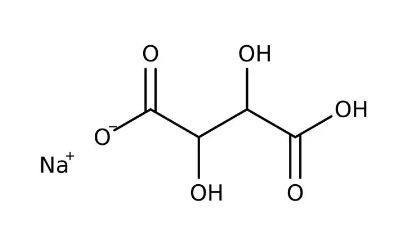
Sodium Hydrogen Tartrate Formula Structure
The Sodium Hydrogen Tartrate formula is C 4 H 5 O 6 Na. It consists of carbon (C), hydrogen (H), oxygen (O), and sodium (Na) atoms. The molecular structure of Sodium Hydrogen Tartrate involves a complex arrangement of these atoms. It contains two carboxylic acid (–COOH) functional groups and hydroxyl (–OH) groups on the carbon backbone, contributing to its acidic properties and ability to act as a buffering agent.
The sodium (Na) atom is bonded to the structure, forming a salt. The compound's structure is intricate and not easily represented in a simple visual format, but it is composed of interconnected carbon, hydrogen, oxygen, and sodium atoms, creating a unique chemical structure.
Sodium Hydrogen Tartrate Formula Physical Properties
Sodium hydrogen tartrate exhibits the following physical properties:
Chemical Formula: Sodium hydrogen tartrate formula is C 4 H 5 O 6 Na.Molecular Weight: Sodium hydrogen tartrate has a molecular weight of 172 g/mol.
Color and Odor: It appears as a white powder with no discernible odor.
Melting Point: The compound has a melting point of 254 degrees Celsius.
pH Range: It typically maintains a pH range between 3.3 and 3.5.
Flame Characteristics: When ignited, it produces a non-sooty flame.
Solubility: Sodium hydrogen tartrate is soluble in water but insoluble in ethanol. It exhibits moderate solubility in calcium tartrate and high solubility in sodium tartrate.
Sodium Hydrogen Tartrate Formula Chemical Properties
Sodium Fluoride is a solid substance that appears white to greenish, devoid of any discernible odor.
Sodium hydrogen tartrate formula is C 4 H 5 O 6 Na. Sodium hydrogen tartrate, when subjected to various chemical reactions, displays the following chemical properties:
Heating in Air: When heated in the presence of air, sodium hydrogen tartrate decomposes, yielding carbonic acid, sodium carbonate, water, and carbon dioxide:
2C 4 H 5 O 6 Na + 5O 2 → H 2 CO 3 + Na 2 CO 3 + 4H 2 O + CO 2
Reaction with Sodium Carbonate: The reaction of sodium hydrogen tartrate with sodium carbonate results in the formation of potassium bitartrate:
C 4 H 5 O 6 Na + K 2 CO 3 → C 4 H 4 O 6 K 2 + CO 2
Formation of Sodium Tartrate: Sodium hydrogen tartrate can also produce sodium tartrate when reacting with sodium carbonate:
C 4 H 5 O 6 Na + Na 2 CO 3 → C 4 H 4 O 6 Na 2 + CO 2
Generation of Sodium Bitartrate: When sodium hydrogen tartrate reacts with sodium hydrogen carbonate, sodium bitartrate is formed:
C 4 H 5 O 6 Na + NaHCO 3 → C 4 H 4 O 6 Na 2 + CO 2
Production of Sodium Hydrogen Tartrate and Hydrochloric Acid: Tartaric acid, when combined with potassium chloride and sodium chloride, yields sodium hydrogen tartrate and hydrochloric acid:
C 4 H 6 O 6 + NaCl + KCl → C 4 H 4 O 6 NaK + HCl
These chemical reactions illustrate the diverse chemical properties of sodium hydrogen tartrate and its ability to undergo transformations under various conditions.
Formation of Sodium Hydrogen Tartrate
Sodium Hydrogen Tartrate formula is C 4 H 5 O 6 Na. Sodium hydrogen tartrate, also known as sodium bitartrate, is synthesized through various chemical reactions:
When tartaric acid is combined with sodium carbonate, sodium hydrogen tartrate is produced:
H 6 C 4 O 6 + Na 2 CO 3 → C 4 H 5 O 6 Na
The reaction of potassium hydrogen tartrate with sodium hydrogen carbonate also yields sodium hydrogen tartrate:
C 4 H 5 O 6 K + NaHCO 3 → C 4 H 4 O 6 KNa
When tartaric acid reacts with sodium chloride, it results in the formation of sodium hydrogen tartrate and hydrochloric acid:
C 4 H 6 O 6 + NaCl → C 4 H 5 O 6 Na + HCl
By combining tartaric acid with both potassium chloride and sodium chloride, sodium hydrogen tartrate and hydrochloric acid are generated:
C 4 H 6 O 6 + NaCl + KCl → C 4 H 4 O 6 NaK + HCl
Another method involves the reaction of tartaric acid with sodium hydrogen carbonate to produce sodium hydrogen tartrate:
C 4 H 6 O 6 + NaHCO 3 → C 4 H 5 O 6 Na
These chemical reactions yield sodium hydrogen tartrate, which can be further utilized in various applications.
Uses of Sodium Hydrogen Tartrate
Sodium hydrogen tartrate finds utility in a range of applications:
Baking Powder: It serves as an essential ingredient in baking powder, contributing to leavening and enhancing the texture of baked goods.
Lacquers: Sodium hydrogen tartrate is utilized in the formulation of lacquers, where it plays a role in enhancing finishes and coatings.
Electrolyte Balance: In the field of healthcare, it is used to help maintain the body's electrolyte balance, particularly in medical and therapeutic contexts.
Antioxidant Properties: It possesses antioxidant properties, effectively scavenging highly reactive species and contributing to oxidative stress reduction.
Buffering Agent: This compound is Used as a buffering agent, making it valuable for preventing spoilage when added to acidic foods.
Ammonium Cation Testing: Sodium hydrogen tartrate is used for testing the presence of ammonium cations, aiding in analytical chemistry and chemical analysis.
Flavoring Agent: This compound is Used as a flavoring agent to add unique taste characteristics to various food products.
Silver Mirror Formation: It plays a key role in the creation of silver mirrors, facilitating a chemical reaction that results in reflective coatings.
Electroplating Baths: Sodium hydrogen tartrate is included in electroplating baths, assisting in the electroplating process to deposit metals onto surfaces.
| Related Links | |
| Hydrazine Formula | Hydrochloric Acid Formula |
| Hydrofluoric Acid Formula | Hydrogen gas Formula |
Sodium Hydrogen Tartrate Formula FAQs
What is the chemical formula for Sodium Hydrogen Tartrate?
What are some common names for Sodium Hydrogen Tartrate?
What are the primary physical properties of Sodium Hydrogen Tartrate?
Is Sodium Hydrogen Tartrate soluble in water?
What is the main application of Sodium Hydrogen Tartrate in baking?










