
Sodium Nitrate Formula: Sodium (Na) is a member of the alkali metal group (Group 1 [IA]) in the periodic table. It exhibits a distinctively soft, silvery appearance. Sodium ranks as the most prevalent alkali metal on Earth, constituting approximately 2.8% of the Earth's composition.
Nitrate is an inorganic compound consisting of one nitrogen atom (N) and three oxygen atoms (O), represented chemically as NO 3 . Generally, nitrate poses no health risks unless it undergoes reduction to nitrite (NO 2 ).
Also Check – Ammonium Nitrate Formula
Sodium Nitrate
Sodium nitrate serves as a common preservative utilized in cured meats, such as bacon, deli meats, and jerky. Its usage has been linked to the development of cardiovascular diseases and diabetes. As a result of these concerns, some products have eliminated this additive.
Sodium nitrate exhibits non-flammable properties and readily dissolves in water, hydrazine, and ammonia. It is soluble in alcohol, slightly soluble in pyridine, and not completely soluble in acetone.
Naturally occurring in mineral deposits, particularly in regions like Peru and Chile, sodium nitrate is a chemical compound. These nitrates are known to accumulate on land due to the deposition of sea fog. They are formed through the oxidation or drying of sea mist and subsequent gravity-induced precipitation of compounds like KNO 3 , Na 2 SO 4 , I, NaNO 3 , and NaCl in the atmosphere. Sodium nitrate finds widespread application in various industries, including the production of flame, glass, food preservatives, fuels, fertilizers, smoke grenades, ceramic enamels, and solid propulsion rockets.
Also Check – Aluminium Nitrate Formula
Sodium Nitrate Formula
The sodium nitrate formula, also referred to as the sodium nitrate formula or cubic nitrate formula, is elucidated in this article. It constitutes an inorganic salt of sodium nitrate (Na) with sodium existing in the cationic form as Na + and nitrate in the anionic form as NO 3 - . The nitrogen atom within the nitrate ion forms two single bonds and one single bond with three oxygen atoms. The chemical or molecular formula representing sodium nitrate is NaNO 3 .
Also Check – Chemistry Formula For Solubility Product
The structure of Sodium Nitrate
Sodium nitrate forms an ionic bond, with one Na+ ion and one NO 3 - ion. The nitrate anion possesses a triangular planar structure, where three oxygen atoms are bonded to a central nitrogen atom. The negative charge within these ions is distributed due to resonance. Consequently, the nitrogen atom carries a charge of +1, while each oxygen atom bears a charge of ⅔. The overall formal charge for NO 3 – is 1.
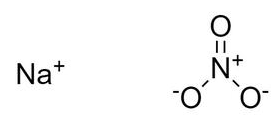
Also Check – Chemical Bonding Formula
Preparation of Sodium Nitrate
In industrial settings, sodium nitrate is synthesized by neutralizing nitric acid using sodium carbonate, sodium bicarbonate, or sodium hydroxide. The chemical reactions for these processes are as follows:
NaHCO 3 + HNO 3 → NaNO 3 + H 2 O + CO 2
Na 2 CO 3 + 2HNO 3 → 2NaNO 3 + CO 2 + H 2 O
NaOH + HNO 3 → NaNO 3 + H 2 O
Alternatively, sodium nitrate can also be obtained through the reaction of sodium hydroxide with ammonium nitrate, rather than using nitric acid. The chemical reaction for this process is:
NH 4 NO 3 + NaOH → NH 4 OH + NaNO 3
Additionally, ammonium nitrate can react with sodium carbonate or sodium bicarbonate as shown below:
Na2CO 3 + 2NH 4 NO 3 → (NH 4 ) 2 CO 3 + 2NaNO 3
NaHCO 3 + NH 4 NO 3 → NH 4 HCO 3 + NaNO 3
Also Read: Boltzmann Constant
Physical Properties of Sodium Nitrate
- Sodium nitrate has a molecular weight calculated from its formula of 84.997 g/mol.
- It possesses a density of 2.257 g/cm³.
- The melting point of sodium nitrate is 308°C, and its boiling point is 380°C.
- It exhibits solubility in water, ammonia, and hydrazine.
- Being an ionic compound with the formula NaNO3, it has slight solubility in pyridine and is insoluble in acetone.
- The crystal structure of sodium nitrate is characterized as triangular and rhombohedral.
- Sodium nitrate salt has the ability to absorb and retain water.
Chemical Properties of Sodium Nitrate
In aqueous solutions, sodium nitrate dissolves into Na+ and NO3– ions, rendering it a potent oxidizing agent that reacts vigorously with reducing agents.
At elevated temperatures, this compound is known to undergo decomposition.
Uses of Sodium Nitrate
Sodium Nitrate finds various applications owing to its favorable properties, including high water solubility, cost-effectiveness, and nitrogen content. Some of its prominent uses are:
Fertilizers: Sodium nitrate is employed in certain fertilizers due to its high water solubility and nitrogen content, which aids in promoting plant growth.
Aqua Regia Hybrid: It is used to create a hybrid form of aqua regia, a powerful solvent mixture. This hybrid is capable of melting gold and is used in various metallurgical processes.
Food Additive: Sodium nitrate acts as a preservative and is commonly utilized as a food additive to extend the shelf life of processed foods.
Fireworks: Sodium nitrate serves as an oxidizing agent in the composition of fireworks, contributing to their colorful and explosive displays.
Instant Cold Compresses: It is found in some instant cold compresses, providing a cooling effect when mixed with water.
Solar Power Plants: In certain solar power plants, sodium nitrate is used as one of the components for storing and transferring heat, facilitating energy capture and storage.
Wastewater Treatment: Sodium nitrate is added to the wastewater in several treatment plants to stimulate the growth of Nitrosomonas bacteria, aiding in the removal of pollutants.
Propellants: It is utilized in certain propellants and can serve as a substitute for potassium nitrate in gunpowder formulations, contributing to its use in various ammunition and pyrotechnic applications.
Sodium Nitrate Formula FAQs
What is Sodium Nitrate used for?
Is Sodium Nitrate harmful to health?
What is the chemical formula of Sodium Nitrate?
How is Sodium Nitrate produced industrially?
Can Sodium Nitrate melt gold?










