
Stearic Acid Formula: Carbon, a nonmetallic element with four valence electrons, holds immense significance for humanity. Its etymology traces back to the Latin word "carbo," signifying charcoal or ember. Despite its modest presence, constituting only 0.025% of the Earth's crust and 0.03% in the atmosphere, carbon plays a pivotal role in various aspects of our lives. Its abundance and its capacity to form polymers under normal conditions make it a fundamental component in all living organisms.
Also Check – Vapor Pressure Formula
Stearic Acid, an extended chain of saturated fatty acids comprising 18 carbon atoms, is occasionally referred to as Octadecanoic acid due to its 18-carbon structure. It is also known as Stereophonic acid. The term "stearic" derives from the Greek word "stear," meaning tallow. The collective term for salts and esters of stearic acid is "stearates."
Stearic acid is naturally present in various animals and plants, typically in a combined form. It is commonly found as a mixed triglyceride or fat alongside numerous other long-chain acids. Additionally, it can exist as an ester of fatty alcohol. Generally, stearic acid occurs in higher quantities in animal fat compared to vegetable fat, although there are exceptions such as cocoa butter and shea butter, which contain substantial amounts of stearic acid.
Also Check – Theoretical Yield Formula
Production of Stearic Acid
Stearic acid is synthesized from carbohydrates, specifically from fats and oils through the process of saponification, which involves the reaction of triglycerides with hot water. Commercially available stearic acid typically consists of a blend of stearic acid, palmitic acid, and a small proportion of palmitoleic acid. While it is feasible to prepare pure stearic acid, its commercial utilization is seldom viable due to several contributing factors.
Also Check – Chemical Bonding Formula
Structure of Stearic Acid
Stearic acid, with the molecular formula C18H36O2 or CH3(CH2)16COOH, is systematically known as Octadecanoic acid.
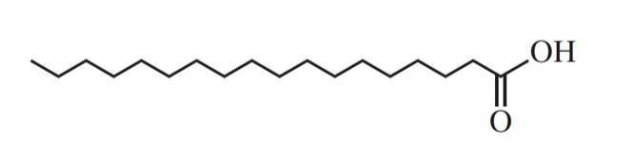
Physical and Chemical Properties of Stearic Acid
- Stearic acid is in the form of a white solid.
- It exhibits an odorless nature.
- Stearic acid has a tallow-like taste.
- Its boiling point is 721°F under normal pressure.
- Under normal pressure, it has a melting point of 156.7°F. This high melting point is a characteristic feature due to its saturated fat nature, requiring significant heat for structural dissociation.
- It is insoluble in water and tends to float on its surface.
- At standard temperature and pressure, stearic acid has a density of 0.86 g/cm³.
- It remains stable at typical room conditions.
- When subjected to temperatures exceeding its boiling point, it decomposes, emitting acrid smoke and irritating fumes.
- Stearic acid possesses a pH of approximately 5.5, classifying it as a weak acid, consistent with other organic acids.
- The molecular mass of stearic acid is 284.48 g/mol.
Also Check – Bleaching Powder Formula
Uses of Stearic Acid
Lubrication: Stearic acid finds utility as a lubricating agent, owing to its soft and smooth texture.
Personal Care Products: It plays a significant role in the production of various personal care items such as detergents, soaps, shampoos, and shaving creams. Esters derived from stearic acid contribute to the pearlescent effect in soaps and shampoos.
Heat Stabilizers: Stearate salts of zinc and calcium serve as effective heat stabilizers in various industrial processes.
Food Additive: Stearic acid is employed as a food additive, enhancing the texture and stability of certain food products.
Food Packaging: It is used in food packaging materials, contributing to their properties.
Electrical Insulators: Stearic acid plays a role in the production of electrical insulators, which are crucial components in electrical systems.
Lead-Acid Batteries: It functions as a negative plate additive in lead-acid batteries, a common type of rechargeable battery.
Candle Manufacturing: Stearic acid is a key ingredient in candle production, influencing their burning characteristics.
Metal Coating: It is used for coating metals like aluminum and iron through electrolytic plating processes, enhancing their surface properties.
Stearic Acid Formula FAQs
What is stearic acid?
Where is stearic acid found naturally?
What is the melting point of stearic acid?
What are the main uses of stearic acid?
Why does stearic acid have a high melting point?










