
Sulfate Ion Formula: Sulfate Ion, also known as sulfate (SO 4 2- ), is a polyatomic molecule composed of one sulfur ion and four oxygen ions. With an empirical formula of SO 4 2- , this molecule carries a net charge of -2 as an anion. Sulfate ions play a significant role in various chemical processes.
Sulfate ions consist of one sulfur (S) atom and four oxygen (O) atoms. Oxygen, denoted by the symbol O with an atomic number of 8, is a highly reactive nonmetal and serves as a potent oxidizing agent. Normally, oxygen exists as a diatomic gas with the molecular formula O 2 , and its valency is 2.
On the other hand, sulfur is represented by the symbol S with an atomic number of 16 and is also a nonmetal. Under typical conditions, sulfur atoms form S8 molecules, comprising eight atoms arranged in a bright yellow crystal structure at room temperature. Sulfur exhibits multiple valencies, with 2 being the predominant valency in S8 molecules.
The formula of the sulfate ion is SO 4 2- , consisting of one sulfur atom and four oxygen atoms, with oxygen having a valency of 2 and sulfur being primarily divalent in S8 molecules.
Also Check – Aluminium Nitrate Formula
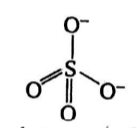
Sulfate Ion Structure
The sulfate ion consists of one sulfur atom and two oxygen atoms arranged in a tetrahedral structure. In this arrangement, the sulfur atom is positioned at the center, surrounded by four equivalent oxygen atoms. Sulfur is in the +6 oxidation state, while oxygen is in the -2 oxidation state. The sulfate ion carries an overall charge of -2, making it unstable and existing in a resonant form.
Within the sulfate ion, double bonds form with two of the oxygen atoms, while single bonds are established with OH molecules. The bond length between sulfur and oxygen atoms in sulfate molecules measures 149 picometers (pm), and these atoms are oriented at an angle of 109.5 degrees.
Preparation of Sulfate Ion
There are two laboratory methods for preparing sulfate ions:
Oxidation of metal sulfides in the presence of oxygen, heat, and a catalyst:
Example 1: 2Cu 2 S + 5O 2 → 2CuSO 4 + 2CuO
Example 2: ZnS + 2O 2 → ZnSO 4
Reaction of metal hydroxides, metal oxides, or pure metals with sulfuric acid:
Example 1: Ba(ClO 3 ) 2 + H 2 SO 4 → 2 HClO 3 + BaSO 4
Example 2: Zn + H 2 SO 4 → ZnSO 4 + H 2
Example 3: Cu(OH )2 + H 2 SO 4 → CuSO 4 + 2 H 2 O
Example 4: CdCO 3 + H 2 SO4 → CdSO 4 + H 2 O + CO 2
Also Read: Boltzmann Constant
Physical Properties of Sulfate Ion
properties of the sulfate ion include:
- IUPAC name: Sulfate
- Melting point: 270.47 degrees Celsius
- Boiling point: 623.89 degrees Celsius
- Molar mass: 96.06 grams per mole
- Most ionic sulfates are highly soluble in water, except for calcium sulfate, strontium sulfate, lead sulfate, and barium sulfate, which have poor solubility.
- Vapor pressure: 0.00791 Pascal
Also Read – Acetone Formula
Chemical Properties of Sulfate Ion
One of the key properties of the sulfate ion is its ability to readily form bonds with metals.
Example: 2NaCl + H 2 SO 4 → Na 2 SO 4 + 2HCl
The Rhodium Sulfate test is the most commonly used method for detecting the presence of sulfate ions.
Example: 2BaCl + H 2 SO 4 → Ba 2 SO 4 + 2HCl
Sulfate ions readily precipitate, forming a white powder upon reaction with certain substances.
Sulfate ions exist as a resonant structure, allowing them to attract metals and form bonds.
Despite having numerous electrons, sulfate ions are often hesitant to bond with other metals due to the oxygen atoms surrounding the sulfate atom, which act as ligands.
The conjugate acid of the sulfate ion is sulfuric acid, while the conjugate base of the sulfate ion is sulfate.
Also Check – Actetamide Formula
Harmful Effects and Safety Measures for Sulfate Ion
- The harmful effects of sulfate ions depend on the specific compounds they form with. There are two main forms of sulfate ions.
- Burning fossil fuels and biomass can lead to increased atmospheric acidity, resulting in acid rain. This occurs because the combustion of sulfate-containing elements releases microscopic aerosol particles into the air.
- These aerosols can cause skin dryness, damage hair, and negatively affect hair due to their negative charge.
Uses and Applications of Sulfate Ion
Sulfate ions play essential roles in various applications:
- Vegetables require sulfate ions for growth and development, often supplied through methods like knapsack sprayers.
- Sulfates are widely used in various industries.
- Gypsum (hydrated calcium sulfate) is used in construction.
- Copper sulfate serves as an electrolyte in laboratory galvanic cells and is a common algaecide.
- Iron sulfate provides iron to living organisms and soil, among other applications.
- Magnesium sulfate is used in therapeutic baths.
- Sulfates are utilized as detergents, emulsifiers, and foaming agents.
- Sulfate compounds are also found in everyday products such as toothpaste, body sprays, lotions, makeup, soaps, and shampoos.
Sulfate Ion Formula FAQs
What is a sulfate ion?
How is a sulfate ion formed?
What is the role of sulfate ions in nature?
What is the significance of sulfate ions in chemistry?
Are all sulfate salts soluble in water?










