
Sulfuric Acid Formula: Sulfuric Acid, also known as Sulphuric Acid, is a mineral acid characterized by its composition of one sulfur atom, four oxygen atoms, and two hydrogen atoms, with the chemical formula H 2 SO 4 . This acid holds significant importance in various commercial applications and goes by alternative names such as Mattling acid, Hydrogen Sulfate, or Vitriol. Exhibiting strong acidity and viscosity, it presents as a colorless, odorless, and corrosive liquid.
Sulfuric Acid finds widespread use in industrial processes. Its applications include metal cleaning, impurity extraction from oil, and the synthesis of chemicals like nitric acid and hydrochloric acid. Additionally, it plays a crucial role in the production of dyes, medicines, detergents, and explosives.
Sulfuric acid's molar mass is calculated at 98.079 g/mol, and it has a density of 1.83 g/cm3. The H 2 SO 4 molecule follows a covalent structure, exhibiting a tetrahedral arrangement and a monoclinic crystal structure. Furthermore, Sulfuric Acid is a component of acid rain due to its solubility in water.
Sulfuric Acid Formula
Sulfuric acid stands out as a highly reactive chemical with a versatile range of applications. It finds utility in various industries, including the manufacturing of lead-based automobile batteries, the production of diverse chemicals, adhesives, explosives, the refining of petroleum, and the treatment of metals. Its widespread use has earned it the moniker "King of Chemicals." The chemical formula for Sulfuric or Sulphuric Acid is H 2 SO 4 .
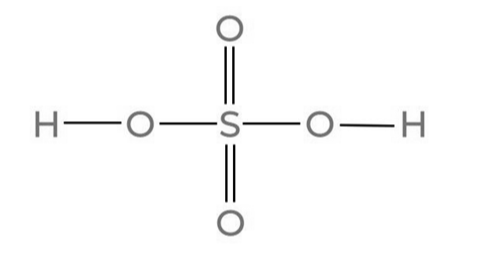
As illustrated below, Sulfuric Acid (H 2 SO 4 ) is a covalent compound composed of a sulfur atom bonded to two oxygen atoms and two hydroxyl molecules (-OH).
Sulfuric Acid Formula Molecular Mass
Sulfuric acid, with the chemical formula H 2 SO 4 , is composed of 2 moles of hydrogen, 1 mole of sulfur, and 4 moles of oxygen atoms in one molecule. Therefore, the molecular mass of H 2 SO 4 is the sum of the masses of two moles of hydrogen, one mole of sulfur, and four moles of oxygen. Given that hydrogen has an atomic mass of 1u, sulfur has an atomic mass of 32u, and oxygen has an atomic mass of 16u, the calculation for the molecular mass of sulfuric acid is as follows: Sulfuric Acid Formula Molecular mass of H 2 SO 4 = Mass of 2 moles of hydrogen atoms + Mass of 1 mole of sulfur + Mass of 4 moles of oxygen atoms Molecular mass of H 2 SO 4 .=Mass of 2 moles of hydrogen atoms+Mass of 1 mole of sulfur+Mass of 4 moles of oxygen atoms
=(2×1)+32+(4×16)
=2+32+64
=98u
Thus, the molecular mass of sulfuric acid is 98u, and its molecular weight is 98 g/mol.
Sulfuric Acid Formula Physical Properties
- H2SO4 is a viscous, thick, colorless, and oily liquid.
- It has a density of 1.84 g/mL, a boiling point of 337 °C, and a melting point of 10 °C.
- Concentrated sulfuric acid is 98% in water and is the most stable form.
- Different concentrations exist for various purposes, such as Battery acid (29–32%), chamber acid (62-70%), and tower acid (78-80%).
- It has a specific gravity of 1.84 at 298 K.
- Turns blue litmus red.
- pH values for sulfuric acid in mmol/l are as follows:
1 mM: 2.75
10 mM: 1.87
100 mM: 1.01
- Highly corrosive, making it dangerous to touch.
Sulfuric Acid Formula Chemical Properties
Complete Dissociation: H2SO4 is a potent acid that fully dissociates into ions in its aqueous solution, represented as:
H2SO4 → H2+ + SO4-2
Oxidizing Agent: Sulfuric Acid serves as a robust oxidizing agent, facilitating the oxidation of other substances by contributing its oxygen atoms. This is evident in reactions such as:
2H2SO4 + C →2H2O + 2SO2 + CO2
2H2SO4 + S → 2H2O + 3SO2
Exothermic Reaction with Water: Sulfuric acid reacts vigorously with water in a highly exothermic process, releasing heat.
Dibasic Nature: Sulphuric Acid is a dibasic acid, releasing two hydrogen ions per molecule.
Hygroscopic Properties: Possessing hygroscopic properties, H2SO4 can attract and regulate moisture from its surroundings. This characteristic makes it an effective dehydrating agent.
Low Volatility: Sulfuric Acid exhibits low volatility, contributing to its role in the preparation of more volatile acids from their respective salts.
Sulfuric Acid Formula Structure
In the molecular configuration of Sulfuric Acid, two oxygen atoms establish double bonds with the sulfur atom, and concurrently, two hydroxyl groups (OH) create single bonds with the sulfur atom. This acid exhibits diprotic characteristics, releasing two protons. The molecular structure is tetrahedral, portraying a covalent nature.
Preparation of Sulfuric Acid
Sulfuric Acid is commonly produced through two widely employed methods: Contact Process: The Contact Process involves three phases in the synthesis of sulfuric acid: Preparation of Sulphur Dioxide: Sulfur or sulphide ores are burned in the presence of air. S(s)+O 2 (g) →SO 2 (g) Reaction to Produce Sulphur Trioxide: Sulphur dioxide reacts with oxygen in the presence of a V2O5 catalyst, resulting in the formation of Sulphur trioxide (SO 3 ). 2SO 2 (g)+O 2 (g) →2SO 3 (g) Conversion to Sulphuric Acid: Sulphur trioxide combines with H 2 SO 4 (Oleum) and undergoes further reaction to yield Sulphuric Acid. SO 3 +H 2 SO 4 (Oleum) →H 2 S 2 O 7 H 2 S 2 O 7 (l)+H 2 O(l) →2H 2 SO 4 (SulfuricAcid) The sulfuric acid obtained through the Contact Process is typically 96–98% pure.Lead Chamber Process
The lead Chamber method stands out as one of the prevalent manufacturing processes, generating approximately 50 to 60 B-grade acids. In this procedure, wet sulfur dioxide, in the presence of nitrogenous oxides (acting as a dynamic impetus), undergoes oxidation with atmospheric oxygen to yield sulfur trioxide. The reaction is expressed as:
2SO 2 +O 2 →2SO 3
Subsequently, the interaction between water and sulfur trioxide leads to the formation of sulfuric acid. This reaction is represented as:
SO 3 +H 2 O →H 2 SO 4
Reactions of Sulfuric Acid
Dissociation: When pure, water-free sulfuric acid is heated, it undergoes dissociation to form sulfur trioxide and water. H 2 SO 4 →SO 3 +H 2 O Acidic Character of Sulfuric Acid: Sulfuric acid exhibits common dibasic acid traits, turning blue litmus paper crimson upon contact. It reacts with sodium hydroxide, forming two groups of salts. NaOH+H 2 SO4 →NaHSO 4 +H 2 O 2NaOH+H 2 SO4 →Na 2 SO 4 +2H 2 O Sulphonating Action of Sulfuric Acid: Concentrated sulfuric acid engages in sulphonation reactions with various organic molecules, such as benzene and toluene, producing sulfonic acids. C 6 H 6 +H 2 SO 4 →C 6 H 5 SO 3 H+H 2 O Precipitation Reactions with Sulfuric Acid: Sulfuric acid induces the formation of insoluble sulfates that precipitate when combined with aqueous solutions of barium, lead, and other salts. H 2 SO 4 +BaCl 2 →BaSO 4 ↓+2HCl Reaction with Sulfur Trioxide: Sulfuric acid reacts with sulfur trioxide to produce oleum, commonly known as fuming sulfuric acid. H 2 SO 4 +SO 3 →H 2 S 2 O 7Sulfuric Acid Uses
Sulfuric acid, recognized as a crucial reagent, serves a myriad of industrial purposes, including:
- Utilized in the synthesis of fertilizers such as ammonium sulfate and lime superphosphate.
- Integral in the manufacturing of colors, explosives, pharmaceuticals, and the production of acids like HCl and HNO3.
- Plays a role in the production of pigments, paints, and polymers.
- Applied in the leather industry.
- Finds use in storage compartments.
- Utilized in various applications within the oil and gas sector.
- A component in the detergent industry.
- Functions as a dehydrator.
- Applied as a reagent in laboratory experiments.
- Used in processes within the paper and textile industries.
- Used for metal cleaning before enameling, electroplating, and galvanizing in metallurgy.
PW Neev Fastrack and Udaan Fastrack Batch are Specially designed for class 9 and 10 students. Enrol Now Online Course of Class 9 Neev Fastrack 2024 and Class 10 Udaan Fastrack 2024 to enhance your chemistry knowledge. and build a strong foundation.
| Related Links | |
| Barium Iodide Formula | Barium Oxide Formula |
| Hydrogen Sulfate Formula | Barium Sulphate Formula |
Sulfuric Acid Formula FAQs
What is the chemical formula for sulfuric acid?
What does the formula H2SO4 represent in terms of its composition?
Is sulfuric acid a strong acid or a weak acid?
What is the molecular mass of sulfuric acid?
What is the structure of sulfuric acid?










